Abstract
Purpose
Recent studies have shown that sodium glucose cotransporter 2 (SGLT2) inhibitors have a favorable effect on cardiovascular events in diabetic patients. However, the underlying mechanism associated with a favorable outcome has not been clearly identified. The purpose of this study was to investigate the effect of tofogliflozin, SGLT2 inhibitor, on systolic and diastolic cardiac function in patients with type 2 diabetes mellitus (T2DM).
Methods
We enrolled 26 consecutive T2DM out-patients on glucose-lowering drugs who initiated tofogliflozin and underwent echocardiography before and ≥ 6 months after tofogliflozin administration. During this period, we also enrolled 162 T2DM out-patients taking other glucose-lowering drugs as a control group. Propensity score analysis was performed to match the patient characteristics. As a result, 42 patients (tofogliflozin group 21 patients and control group 21 patients) were finally used for analysis. Left ventricular systolic function was assessed by measuring 2D-echocardiographic left ventricular ejection fraction (LVEF) and diastolic cardiac function by pulsed wave Doppler-derived early diastolic velocity (E/e′).
Results
There were no significant differences in patient characteristics and echocardiographic parameters at baseline. The change in LVEF from baseline to follow-up was 5.0 ± 6.9% in the tofogliflozin group and − 0.6 ± 5.5% in the control group; difference significant, p = 0.006. The change in E/e′ was − 1.7 ± 3.4 in the tofogliflozin group and 0.7 ± 4.1 in the control group; difference significant, p = 0.024.
Conclusions
In addition to conventional oral glucose-lowering drugs, additional tofogliflozin administration had a favorable effect on left ventricular systolic and diastolic function in patients with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increased risk of cardiovascular disease including heart failure is observed in type 2 diabetes mellitus (T2DM) [1, 2]. Diabetes mellitus (DM) induces left ventricular (LV) dysfunction which is independent of glycemic control, hypertension, and coronary artery disease, and DM-related LV dysfunction is associated with increased risk of cardiovascular disease [3, 4]. Underlying pathogenic mechanisms associated with DM-related LV dysfunction is considered to be myocardial fibrosis, necrosis, apoptosis due to hyperglycemia, oxidative stress, and chronic inflammation [5, 6]. Unfortunately, conventional glucose-lowering therapies have shown insufficient results in improving LV dysfunction and preventing cardiovascular disease, because development of cardiovascular disease is related not only to glycemic control but also to combined effects of T2DM [7,8,9,10].
Sodium glucose cotransporter 2 (SGLT2) inhibitors emerged as a glucose-lowering drug via urinary glucose excretion. SGLT2 inhibitors have multifactorial effects on atherosclerotic risk factors such as reducing blood pressure and body weight, and improving dyslipidemia [11,12,13]. In addition, T2DM patients taking SGLT2 inhibitors showed less incidence of cardiovascular disease and heart failure in EMPA-REG and CANVAS trials [14, 15]. However, the underlying mechanism associated with a favorable effect of SGLT2 inhibitors on cardiac function is not well known. Accordingly, we investigated the effect of tofogliflozin, SGLT2 inhibitor, on systolic and diastolic cardiac function in patients with T2DM.
Methods
Patient Selection
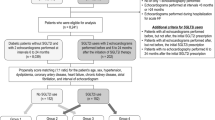
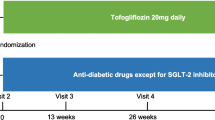
We studied 26 consecutive T2DM out-patients taking glucose-lowering drugs and initiated tofogliflozin (20 mg/day) between February 2015 and December 2017. All patients fulfilled the following inclusion criteria; age ≥ 20 years, no previous SGLT2 inhibitor administration, and performed echocardiography before initiation of tofogliflozin (baseline) and ≥ 6 months after administration of tofogliflozin (follow-up). During this period, 162 out-patients with T2DM taking other glucose-lowering drugs and fullfilling the inclusion criteria were used as a control group. During the study periods, patients having cardiovascular events including myocardial infarction or heart failure hospitalization were not included in this study. Propensity score analysis was performed to match the patient characteristics. As a result, 42 patients (tofogliflozin group 21 patients and control group 21 patients) were finally used for analysis.
Evaluated Variables
Clinical characteristics including demographic characteristics, comorbidity, prescribed medicine, laboratory parameters, and echocardiographic findings were obtained at baseline and at follow-up. Baseline medications were obtained from the medical record. Echocardiography was performed with commercially available ultrasound system using Vivid 7 or Vivid E9 GE Medical System. Standard transthoracic and Doppler echocardiographic parameters were examined according to the current guideline of the American Society of Echocardiography/European Association of Cardiovascular Imaging [16]. Systolic cardiac function was evaluated by measuring left ventricular ejection fraction (LVEF) using Modified Simpson method. Early diastolic (E) and atrial wave (A) flow velocities of the mitral valve and E wave deceleration time were measured by pulsed wave Doppler echocardiography from the apical four-chamber view. Spectral pulsed wave Doppler-derived early diastolic velocity (e′) was calculated by averaging the septal and lateral mitral annulus. E/e′ was used as a diastolic cardiac function [17, 18]. All classic views were analyzed by a physician who was board certified in echocardiography. Follow-up information was obtained from the medical record. Informed consent was obtained through an opt-out procedure from all participants. The study protocol was approved by the ethics committees of Kansai Medical University Medical Center.
Statistical Analyses
Continuous variables are presented as medians with interquartile ranges or as means ± standard deviations. Categorical variables are presented as numbers and percentages. Differences between the two groups were analyzed using the unpaired t test or Mann-Whitney U test for continuous variables and the chi-square test for categorical variables. Differences between baseline and follow-up data were conducted using the paired t test. A p value < 0.05 was considered significant. To minimize various biases between the two groups, propensity score matching method was conducted. Propensity score was calculated by multivariate logistic regression analysis using seven variables: age, sex, serum creatinine level, HbA1c at baseline, hypertension, body mass index, and number of glucose-lowering drugs. All seven variables are reported as influencing factors for systolic and diastolic cardiac function and to avoid possible differences in the severity of diabetic status between the two groups, the number of glucose-lowering drugs was included in the propensity matching analysis [19]. JMP 13.0.0 software (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
Results
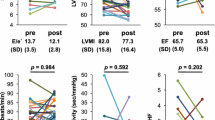
Median follow-up periods were 8.0 (6.5–10.9) months in the tofogliflozin group and 9.1 (6.1–10.3) months in the control group (p = 0.414). Patient characteristics are shown in Table 1. There were no significant differences in comorbidities and oral medications between the two groups. Baseline and follow-up laboratory and echocardiographic parameters are shown in Tables 2 and 3. There was no significant difference in blood pressure between the two groups during the study period. Blood pressure-lowering drugs including angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker, and beta blocker were prescribed continuously with no change in the dose, whereas glucose-lowering drugs were adjusted in each patient except tofogliflozin. There were no significant differences in baseline laboratory and echocardiographic parameters between the two groups but the hemoglobin level at follow-up was significantly higher in the tofogliflozin group than the control group (tofogliflozin 15.5 (14.2–16.6) g/dL vs. control 13.8 (12.2–14.6) g/dL, p = 0.002). The majority of the patients in this study had preserved LVEF which is common in out-patient DM clinic. At follow-up, there were no significant differences in echocardiographic parameters between the two groups, except E/e′ at follow-up (tofogliflozin 10.6 (8.1–14.8) vs. control 13.2 (11.7–17.7), p = 0.021). LVEF improved significantly in tofogliflozin group (baseline 55 ± 14% to follow-up 60 ± 12%, p = 0.003, Fig. 1a), whereas LVEF did not change significantly in the control group (baseline 57 ± 18% to follow-up 56 ± 18%, p = 0.641, Fig. 1b). The change in LVEF from baseline to follow-up was significantly greater in the tofogliflozin group compared to the control group (5.0 ± 6.9% vs. − 0.6 ± 5.5%, p = 0.006, Fig. 1c). E/e′ improved significantly in the tofogliflozin group (baseline 13.0 ± 4.8 to follow-up 11.4 ± 3.6, p = 0.037, Fig. 2a), whereas E/e′ did not change significantly in the control group (baseline 13.9 ± 4.6 to follow-up 14.6 ± 5.3, p = 0.530, Fig. 2b). The change in E/e′ from baseline to follow-up was significantly larger in the tofogliflozin group compared to the control group (− 1.7 ± 3.4 vs. 0.7 ± 4.1, p = 0.024, Fig. 2c).
Discussion
Several clinical studies have reported the effect of SGLT2 inhibitors on cardiac function in T2DM. A retrospective small study (n = 10) showed that empagliflozin reduced LV mass index and improved lateral e′ in patients with T2DM [20]. A prospective single-arm study (n = 37) showed that canagliflozin reduced LV mass index and improved diastolic function in T2DM patients [21]. Another prospective single-arm study (n = 58) showed that dapagliflozin improved E/e′ as well as LV mass index in T2DM patients with chronic heart failure [22]. These three single-arm and small in number studies showed a favorable effect of SGLT2 inhibitors on diastolic function in T2DM patients. However, none of these studies looked into the effect of SGLT2 inhibitor on systolic function. This is the first study to demonstrate that tofogliflozin significantly improved systolic and diastolic cardiac function after ≥ 6-month period compared to the propensity matched control group.
Urine glucose and sodium excretion are two major factors contributing to osmotic diuresis induced by SGLT2 inhibitors. Osmotic diuresis results in reduction of intravascular fluid and decrease in cardiac preload [23, 24]. Furthermore, reduction of intravascular fluid has a blood pressure-lowering effect which leads to decrease in cardiac afterload [25, 26]. Considering the fact that blood pressure did not change after tofogliflozin administration in our study, reduction of LV filling pressure was the major factor associated with a decrease in cardiac workload and hence, improvement of LVEF and reduction of E/e′ [27]. Reduction of intravascular fluid and fall in blood pressure may lead to augmentation of the renin-angiotensin-aldosterone system and sympathetic nervous system activities. However, Cherney et al. reported that SGLT2 inhibitors induced renin-angiotensin-aldosterone system inhibition in type 1 diabetes mellitus [28]. Moreover, prospective study showed SGLT2 inhibitors did not exacerbate autonomic function such as baroreflex sensitivity and heart rate variability [21]. These data indicate that SGLT2 inhibitors cause a reduction of intravascular fluid and blood pressure without augmenting the renin-angiotensin-aldosterone system and sympathetic nervous system. Although the underlying mechanism is not well known, one of the possible causes of this phenomenon is considered to be related to plasma osmolality. Plasma osmolality is comprised of serum sodium, glucose, and blood urea nitrogen. Since loop diuretics mainly work to increase urinary sodium excretion, they strongly affect plasma osmolality because serum sodium is the main determinant of plasma osmolality. As a result of rapid intravascular fluid reduction, loop diuretics cause augmentation of the renin-angiotensin-aldosterone system and sympathetic nervous system. In contrast, urinary sodium excretion caused by SGLT2 inhibition is transient [29] and the SGLT2 inhibitor works mainly on urinary glucose excretion, which has a small effect on plasma osmolality. Therefore, SGLT2 inhibition may induce reduction of intravascular fluid and a fall in blood pressure without augmentation of renin-angiotensin-aldosterone system and sympathetic nervous system activities. Recently, Cohen et al. investigated the effect of empagliflozin on cardiac function using cardiac magnetic resonance in 25 T2DM and found that empagliflozin significantly reduced LV end-diastolic volume compared to pre-treatment [30]. Our study is consistent with Cohen et al. in that plasma volume reduction rather than structural remodeling resulted in the functional improvement (LV systolic and diastolic function) after 6-month tofogliflozin treatment.
It is difficult to determine the direct drug effect of SGLT2 on the myocardium because the SGLT2 receptor is not expressed in the human heart [31]. Conversely, SGLT1 is highly expressed in the human heart [32]. SGLT1 contributes to glucose uptake during myocardial ischemic injury, and experimental studies indicated that cardiac SGLT1 expression and function are accelerated in diabetic and ischemic cardiomyopathies [33]. Moreover, exacerbation of cardiac dysfunction and ischemic reperfusion injury was caused by SGLT1 blockade in a mice model [32, 34]. Among SGLT2 blockers, tofogliflozin has the highest SGLT2 selectivity with 2900-fold greater selectivity than SGLT1 [35]. Therefore, low selectivity of tofogliflozin to the SGLT2 receptor may contribute to a favorable effect on cardiac function in T2DM.
Experimental studies have suggested that SGLT2 inhibitors reduce myocardial profibrotic signaling which prevents myocardial fibrosis and improves diastolic function [36,37,38]. In addition to the hemodynamic effect of tofogliflozin, these underlying molecular mechanisms may potentially mediate a favorable effect of tofogliflozin on cardiac function. Thus, a significant increase in LVEF and decrease in E/e′ after tofogliflozin administration indicates that hemodynamic and molecular effects of tofogliflozin played an important role in the improvement of systolic and diastolic function in T2DM patients.
Clinical Implication
Although DM-related LV dysfunction is an important cause of cardiovascular events including heart failure [39,40,41], conventional glucose-lowering drugs have shown insufficient results in preventing the progression of LV dysfunction. This study showed tofogliflozin improved systolic and diastolic LV function, indicating that tofogliflozin treatment should be performed as early as possible not only as glucose-lowering therapy but also to prevent progression of LV dysfunction in T2DM.
Limitation
There are three limitations in this study. First, our study was an observational study including small number of patients. Although further prospective study is required with a larger number of patients, this is the first study to show a significant improvement of systolic and diastolic function by adding tofogliflozin in T2DM patients. Second, prescription of glucose-lowering drugs including tofogliflozin was done by the attending physician. Nonetheless, we believe that propensity-matching analysis used in this study avoided patient selection bias. Finally, precise BMI values at 6-month follow-up was not measured. Although the change in body weight was not evaluated, we found a significant improvement of LV function after 6 months of tofogliflozin treatment.
Conclusions
In addition to conventional oral glucose-lowering drugs, additional administration of tofogliflozin had a favorable effect on LV systolic and diastolic function in patients with T2DM.
References
Vazquez-Benitez G, Desai JR, Xu S, Goodrich GK, Schroeder EB, Nichols GA, et al. Preventable major cardiovascular events associated with uncontrolled glucose, blood pressure, and lipids and active smoking in adults with diabetes with and without cardiovascular disease: a contemporary analysis. Diabetes Care. 2015;38:905–12.
Chen G, McAlister FA, Walker RL, Hemmelgarn BR, Campbell NR. Cardiovascular outcomes in Framingham participants with diabetes: the importance of blood pressure. Hypertension. 2011;57:891–7.
From AM, Scott CG, Chen HH. Changes in diastolic dysfunction in diabetes mellitus over time. Am J Cardiol. 2009;103:1463–6.
van den Hurk K, Alssema M, Kamp O, Henry RM, Stehouwer CD, Smulders YM, et al. Independent associations of glucose status and arterial stiffness with left ventricular diastolic dysfunction: an 8-year follow-up of the Hoorn Study. Diabetes Care. 2012;35:1258–64.
Ernande L, Derumeaux G. Diabetic cardiomyopathy: myth or reality? Arch Cardiovasc. 2012;105:218–25.
Zhang X, Chen C. A new insight of mechanisms, diagnosis and treatment of diabetic cardiomyopathy. Endocrine. 2012;41:398–409.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26.
Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89.
Fischer M, Baessler A, Hense HW, et al. Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. Eur Heart J. 2003;24:320–8.
Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, et al. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol. 2004;43:1432–8.
Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34:2015–22.
Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–31.
Chilton R, Tikkanen I, Hehnke U, Woerle HJ, Johansen OE. Impact of empagliflozin on blood pressure in dipper and non-dipper patients with type 2 diabetes mellitus and hypertension. Diabetes Obes Metab. 2017;19:1620–4.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70.
Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94.
Oh JK, Park SJ, Nagueh SF. Established and novel clinical applications of diastolic function assessment by echocardiography. Circ Cardiovasc Imaging. 2011;4:444–55.
Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–18.
Verma S, Garg A, Yan AT, Gupta AK, al-Omran M, Sabongui A, et al. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME Trial? Diabetes Care. 2016;39:e212–3.
Matsutani D, Sakamoto M, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol. 2018;17:73.
Soga F, Tanaka H, Tatsumi K, Mochizuki Y, Sano H, Toki H, et al. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol. 2018;17:132.
Pham SV, Chilton RJ. EMPA-REG OUTCOME: The Cardiologist’s point of view. Am J Cardiol. 2017;120:S53–8.
Rajasekeran H, Lytvyn Y, Cherney DZ. Sodium-glucose cotransporter 2 inhibition and cardiovascular risk reduction in patients with type 2 diabetes: the emerging role of natriuresis. Kidney Int. 2016;89:524–6.
Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diabetes Vasc Dis Res. 2015;12:90–100.
Bouchi R, Terashima M, Sasahara Y, Asakawa M, Fukuda T, Takeuchi T, et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: a pilot study. Cardiovasc Diabetol. 2017;16:32.
Lioudaki E, Androulakis ES, Whyte M, Stylianou KG, Daphnis EK, Ganotakis ES. The effect of sodium-glucose co-transporter-2 (SGLT-2) inhibitors on cardiometabolic profile; beyond the hypoglycaemic action. Cardiovasc Drugs Ther. 2017;31:215–25.
Cherney DZ, Perkins BA, Soleymanlou N, et al. Sodium glucose cotransport-2 inhibition and intrarenal RAS activity in people with type 1 diabetes. Kidney Int. 2014;86:1057–8.
Heise T, Jordan J, Wanner C, Heer M, Macha S, Mattheus M, et al. Pharmacodynamic effects of single and multiple doses of empagliflozin in patients with type 2 diabetes. Clin Ther. 2016;38:2265–76.
Cohen ND, Gutman SJ, Briganti EM, Taylor AJ. The effects of empagliflozin treatment on cardiac function and structure in patients with type 2 diabetes – a cardiac MR study. Intern Med J 2019 (In press).
Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–94.
Kashiwagi Y, Nagoshi T, Yoshino T, Tanaka TD, Ito K, Harada T, et al. Expression of SGLT1 in human hearts and impairment of cardiac glucose uptake by phlorizin during ischemia-reperfusion injury in mice. PLoS One. 2015;10:e0130605.
Banerjee SK, McGaffin KR, Pastor-Soler NM, Ahmad F. SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc Res. 2009;84:111–8.
Connelly KA, Zhang Y, Desjardins JF, Thai K, Gilbert RE. Dual inhibition of sodium–glucose linked cotransporters 1 and 2 exacerbates cardiac dysfunction following experimental myocardial infarction. Cardiovasc Diabetol. 2018;17:99.
Suzuki M, Honda K, Fukazawa M, Ozawa K, Hagita H, Kawai T, et al. Tofogliflozin, a potent and highly specific sodium/glucose cotransporter 2 inhibitor, improves glycemic control in diabetic rats and mice. J Phamacol Exp Ther. 2012;341:692–701.
Hammoudi N, Jeong D, Singh R, Farhat A, Komajda M, Mayoux E, et al. Empagliflozin improves left ventricular diastolic dysfunction in a genetic model of type 2 diabetes. Cardiovasc Drugs Ther. 2017;31:233–46.
Habibi J, Aroor AR, Sowers JR, Jia G, Hayden MR, Garro M, et al. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc Diabetol. 2017;16:9.
Gupte M, Umbarkar P, Lal H. Mechanistic insights of empagliflozin-mediated cardiac benefits: nearing the starting line: editorial to: “Empagliflozin improves left ventricular diastolic dysfunction in a genetic model of type 2 diabetes” by N. Hammoudi et al. Cardiovasc Drugs Ther. 2017;31:229–32.
Ryden L, Grant PJ, Anker SD, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34(39):3035–87.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–239.
González-Vilchez F, Ayuela J, Ares M, Pi J, Castillo L, Martin-Durán R. Oxidative stress and fibrosis in incipient myocardial dysfunction in type 2 diabetic patients. Int J Cardiol. 2005;101(1):53–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethical Approval
The study was approved by the ethical committee of the Kansai Medical University Medical Center and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study using opt-out procedure.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Otagaki, M., Matsumura, K., Kin, H. et al. Effect of Tofogliflozin on Systolic and Diastolic Cardiac Function in Type 2 Diabetic Patients. Cardiovasc Drugs Ther 33, 435–442 (2019). https://doi.org/10.1007/s10557-019-06892-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-019-06892-y