Abstract
Background
Heart failure (HF) is the end stage of many heart diseases, and ischemic heart disease (IHD) is the primary cause. Yiqi Fumai lyophilized injection, a contemporary Chinese medicine preparation, widely used in the treatment of IHF patients, shows clinical efficacy on improving symptoms and cardiac function, but the quality of the current literature does not address multiple important issues. This article describes a protocol for assessment of complementary treatment with Yiqi Fumai lyophilized injection in acute decompensated IHD.
Methods
The protocol is designed as a multicenter randomized controlled trial to assess the efficacy and safety of complementary treatment with Yiqi Fumai lyophilized injection on acute decompensated IHD. This trial will be carried out in 37 hospitals in China and expected to enroll 666 inpatients with acute decompensated IHF due to coronary heart disease. On the basis of standardized western medications, patients are randomized to either the treatment group (250 ml 5% glucose / sodium injection + 5.2 g Yiqi Fumai lyophilized injection) or the control group for 7 days and follow-up for 30 ± 3 and 60 ± 3 days. The primary outcome is change in brain natriuretic peptide (BNP) concentrations. The secondary outcomes are composite endpoint, left ventricular ejection fraction, blood troponin T/I, cardiothoracic ratio, life quality scale, scores of the four traditional Chinese medicine (TCM) diagnostic methods.
Discussion
Standardized western medications together with TCM have been extensively used in China and have developed into a comprehensive treatment model. The trial will provide clinical research evidence for application of complementary treatment with intravenous Yiqi Fumai lyophilized injection on decompensated IHF.
Trial Registration
This study protocol has been listed in the Chinese Clinical Trial Registry (registration number: ChiCTR-IPR-15007396, http://www.chictr.org.cn/showproj.aspx?proj=12370) on November 6, 2015.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) is the end stage of many heart diseases. The high hospital admission rate and low 5-year survival rate in HF are comparable to malignant tumor and have seriously threatened the health of people. Studies have shown that ischemic heart disease (IHD) is the primary cause of heart failure [1]. HF guidelines have constantly been updated, so the treatment for ischemic HF patients is now more standardized. Thus, the clinical prognosis has been improved. Even so, the readmission rate of HF patient remains high and the heart function continues to deteriorate [2].
Standardized western medications together with traditional Chinese medicine (TCM) have been extensively used in China and have developed into a comprehensive treatment model. Intravenous Yiqi Fumai lyophilized injection, a contemporary Chinese medicine preparation, has often been used on ischemic HF patients. Small sample size clinical studies have suggested that intravenous Yiqi Fumai lyophilized injection used along with standardized western medications can further improve clinical efficacy by improving symptoms and cardiac function of patients [3]. However, the quality of the current literatures has limitations. This study aims to evaluate the efficacy and safety of using intravenous Yiqi Fumai lyophilized injection to treat acute exacerbation ischemic HF through central randomization and parallel controlled trial design.
Methods/Design
Study Objectives
To prove complementary treatment with intravenous Yiqi Fumai lyophilized injection based on standardized western treatment can reduce brain natriuretic peptide (BNP) concentratrions, and the incidence of 2-month cardiovascular events in acute decompensated ischemic HF patients.
Design Overview
The study is designed as a multicenter randomized controlled trial. To avoid regional differences, 37 hospitals from different regions of China will be participating in this trial. A research assistant at each participating unit will be responsible for data collection. Data management and statistical analysis will be performed by the statistical department in First Teaching Hospital of Tianjin University of Traditional Chinese Medicine. This study is conducted in accordance with the Declaration of Helsinki and has been approved by the ethics committee in First Teaching Hospital of Tianjin University of Traditional Chinese Medicine (Ethics approval number: TYLL2015[K] No.004). The study has also been registered with the Chinese Clinical Trial Registry (ChiCTR-IPR-15007396). All study subjects have to sign informed consent prior to randomization.
Patient Selection Criteria
Six hundred sixty-six subjects from 37 participating level-A hospitals who satisfy both the inclusion and exclusion criteria will be recruited into the study (inclusion and exclusion criteria in Table 1). Basic information of the subjects will be collected (including sex, age, medical history, medication), severity of disease (including classification of cardiac function, scores from the four TCM diagnostic methods and life quality scale), and physical and chemical examinations (left ventricular ejection fraction, cardiothoracic ratio, blood troponin T/I, etc.).
Randomization Process
Interactive Web Response System (IWRS), a central randomization management system, will be employed for randomized group allocation. Through a dynamic randomization process, the patients are either allocated to the treatment group (YQFM group) or the control group in the ratio of 1:1. When a patient satisfies the inclusion and exclusion criteria, the research assistant will enter the IWRS, input the required information as prompted by the system and a study subject identification number (SSID) will be produced. According to the SSID, there will be a selection performed based on stratification factors such as classification of the cardiac function, age, and sex. Then, the randomization system will generate the randomization code and the information of the study group allocated to the study subject.
Interventions
This study has two phases, the intervention phase and follow-up phase. With reference to the China HF diagnosis and treatment guidelines 2014 [4], patients recruited into the study will be provided standardized western medications (V0 phase). If there are no contraindications, western medicine such as anti-platelets, statins, diuretics, angiotensin-converting-enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARB), β-blockers, spironolactone and digitalis should be used. Study subjects in the control groups will only receive standardized western medications, while the patients in the treatment group will receive eight bottles of intravenous Yiqi Fumai lyophilized injection (0.65 g/bottle) in addition to standardized western medications. Each bottle of Yiqi Fumai will be added into 250 ml of 5% glucose/sodium chloride intravenous infusion and administered at a rate of 60 ml/h (infusion pump recommended) once daily. The patients will receive the intervention for 7 days and other Chinese medicine is to be avoided. After the 7-day intervention, the patients will enter a 2-month follow-up phase. Patients are to follow-up on 8 days (V1), 30 ± 3 days (V2), and 60 ± 3 days (V3) after allocated into the study groups (see Table 2 for details).
Intervention should be administered for 7 days unless the occurrence of certain adverse events that has contraindications. In the presence of comorbidities and complications, western medicine can be given with reference to the relevant guidelines. Researchers are to document all the latest drugs that the patients are taking. When necessary, patients should seek emergency medical help.
Outcomes
The primary outcome is BNP level. The secondary outcomes are composite endpoint (all-cause death, HF emergency/readmission, acute coronary syndrome, revascularization, malignant arrhythmia, cardiogenic shock, stroke, and pulmonary embolism), left ventricular ejection fraction (LVEF), blood troponin T/I (TNT/TNI), cardiothoracic ratio, life quality scale, scores of the four TCM diagnostic methods, etc.
Follow-up Protocol
All recruited patients will be scheduled for follow-up visits (V1, V2, and V3) after allocation. Acquisition of BNP, echocardiogram, TNT/TNI, cardiothoracic ratio, and scores of the four TCM diagnostic methods will be evaluated before allocation of group, and V1 and V3. Life quality scale will only be evaluated before group allocation and V3 after group allocation.
Sample Size Estimation
Sample size was calculated based on 30% decrease in BNP after treatment. It was hypothesized that the decrease in BNP > 30% in 60% of those given standard western medicine treatment and 70% of those given both Chinese medicine and western medicine. The subjects were arranged in the ratio of 1:1 into the treatment group or control group. There should be 333 patients in each group based on one-tailed difference test formula (significance level α is 0.05 and power (1-β) is 0.80 in one-sided test) and less than 20% of the incomplete rate.
Statistical Approach
Statistical analysis will be performed using SAS 9.1 software. Confidence interval was used to analyze if the main outcome measure has superior efficacy in one group over the other. Other outcome measures were analyzed using two-tailed test, with significance level α = 0.05 (otherwise stated). Numerical data are described by mean, standard deviation, maximum value, and minimum value. Categorical data are described by frequency, percentage, and composition ratio.
Safety Assessment
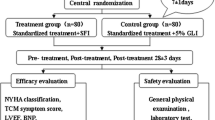
Safety assessment was based on vital signs, laboratory examinations, and adverse events. Vital signs documented before and after treatment will include blood pressure, heart rate, etc. Laboratory examinations to be performed include blood and urine routines, electrolytes, liver and kidney profile, and electrocardiogram (detailed monitoring schedule in Table 2). Adverse events, especially the severe adverse events, are to be reported to the research group committee within 24 h. The study design refers to Fig. 1.
Discussion
Intravenous Yiqi Fumai lyophilized injection originates from the ancient formula shengmai powder and is widely used in the treatment of acute decompensated ischemic HF in China. The raw materials of the injection are red ginseng, liriope, schisandra, and chinensis; excipients are meglumine and mannitolm, are manufactured using modern technology, and contain ginsenosides, ophiopogon polysaccharide, and other active ingredients [5, 6]. Modern pharmacological research shows that ginseng can excite the cerebral cortex and vascular motorium, improve the ability of adrenal cortex secretion, and inhibit the activity of Na+-K+-ATP enzyme to promote calcium influx, strengthen myocardial contraction, and improve heart function [7]; Ophiopogon japonicus polysaccharides can protect myocardial cells, improve blood flow, and have the function of increasing free radicals and scavenging oxygen free radicals caused by inhibition of myocardial ischemia to dilate coronary artery and increase coronary blood flow [8]; Schisandra chinensis can significantly improve the common hypoxia tolerance of animals and has a strong protective effect on animals’ acute myocardial ischemia [9].
A systematic review [3] included a total of 18 articles and 1939 patients, which used Intravenous Yiqi Fumai lyophilized injection along with conventional western medications in the assessment of the curative effect and safety on HF, shows that Intravenous Yiqi Fumai lyophilized injection along with conventional western medications may further improve symptoms and cardiac function, and improve clinical effects, which make a fundamental contribution for further evaluation of the clinical efficacy of intravenous Yiqi Fumai lyophilized injection in the treatment of HF. In order to objectively evaluate the efficacy of intravenous Yiqi Fumai lyophilized injection on HF, a proper outcome is essential. BNP is one kind of heart peptide hormones, mainly secreted by the brain and ventricles, and has the function of promoting diuresis, vasodilating, inhibiting the release of catecholamine by sympathetic nervous system, and reducing the vasoconstriction, as well as raised blood pressure caused by angiotensin-aldosterone [10]. During acute exacerbated phase of HF, ventricular pressure overload and blood volume increases, thus stimulates the myocardial cells to synthesize BNP and releases it into the blood. BNP level is a sensitive indicator of HF damage in its early stage and it is used clinically to assess the severity of the condition and effectiveness of the treatment [11, 12]. Studies have also shown that decrease in BNP level in HF patients would indicate that the risk of readmission and death is reduced [13,14,15]. Thus, BNP is a suitable primary outcome measure for our research.
In recent years, guidelines for the diagnosis and treatment of HF have been updated continuously, and the overall treatment level has been improving. However, further reduction of death and rehospitalization, improvement of clinical symptoms, exercise tolerance, quality of life, and reduction of financial burden remain the constantly goal in clinical treatment.
Conclusions
The aim of ACT-ADIHF is to bring patients long-term benefits by showing complementary treatment with intravenous Yiqi Fumai lyophilized injection may reduce BNP level, the incidence of 2-month cardiovascular events, and improve cardiac function and quality of life in acute decompensated ischemic HF patients.
Trial Status
Since 23 October 2015, this study has recruited 560 patients, and 450 patients have completed 2 months of follow-up.
References
Fox KF, Cowie MR, Wood DA, Coats AJ, Gibbs JS, Underwood SR, et al. Coronary artery disease as the cause of incident heart failure in the population [J]. Eur Heart J. 2001;22(3):228–36.
Zhao Z, Mao J, Wang X, et al. Multicentre investigation of TCM syndromes in acute exacerbation of chronic heart failure [J]. J Tradit Chin Med. 2013;54(12):1038–42.
Wang X, Ma N, Hou Y, et al. Meta-analysis of curative effect of injection of Yiqi Fumai lyophilized injection and western medicine on chronic heart failure [J]. J Tradit Chin Med. 2016;57(5):391–5.
Chinese Medical Association Cardiovascular Branch, Chinese Journal of Cardiology Editorial Board [J]. 2014 Guidelines for Diagnosis and Treatment of Chronic Heart Failure in China. Chin J Cardiovasc Dis. 2014;42(2):98–122.
Li H, Wei G, Yin Y, et al. Research progress of ginseng protecting vascular endothelial cells [J]. Chin J Gerontol. 2015;35(20):5957–60.
Yuan C-L, Sun L, Yuan S-T, et al. Pharmacological activities and possible mechanism of effective components in Ophiopogonis radix [J]. Chin J New Drugs. 2013;22(21):2496–502.
Wang W, Su G, Hu W, et al. Research progress in pharmacological effects of ginsenoside on cardiovascular diseases in last decade [J]. Chin Tradit Herb Drugs. 2016;47(20):3736–41.
Xu D, Feng Y, Zhou Y, et al. Active components of polysaccharide of Ophiopogon japonicus on acute myocardial ischemia [J]. Chin Tradit Patent Med. 2004;26(10):832–7.
Qi Y, Zhao C. Progress of modern pharmacology of Schisandra chinensis[J]. Guide China Med. 2011;9(26):43–4.
Wang H. Laboratory Diagnosis. 2nd ed. Beijing: People’s Health Publishing House; 2010. p. 167–90.
Chen Z. Clinical application of B type natriuretic peptide in diagnosis and treatment of heart failure [J]. Chin Pract Med. 2013;8(28):127–8.
Li P-D. Value of B-type natriuretic peptide testing in diagnosis of heart failure [J]. Prev Treat Cardiovasc Dis Knowl. 2015;7:82–4.
Silver MA, Maisel A, Yancy CW, McCullough PA, Burnett JC, Francis GS, et al. BNP consensus panel 2004: a clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular disease [J]. Congest Heart Fail. 2004;10(3):1–30.
Gustafsson F, Steensgaard-Hansen F, Badskjaer J, et al. Diagnostic and prognostic performance of N-terminal ProBNP in primary care patients with suspected heart failure [J]. J Card Fail. 2005;11(5):S15–20.
Masson S, Latini R, Anand IS, Vago T, Angelici L, Barlera S, et al. Direct comparison of B type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the valsartan heart failure (Val-HeFT) data [J]. Clin Chem. 2006;52(8):1528–38.
Acknowledgements
The authors would like to thank the following participating organizations: First Teaching Hospital of Tianjin University of TCM, Second Affiliated Hospital of Tianjin University of TCM, Tianjin Hospital of ITCWM Nankai Hospital, The Second Hospital of Tianjin Medical University, Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital, Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University, The First Affiliated Hospital of Anhui University of Chinese Medicine, Wuhu Hospital of Traditional Chinese Medicine, Gansu University of Chinese Medicine, Yinchuan Hospital of Traditional Chinese Medicine, The First Affiliated Hospital of Changchun University of Chinese Medicine, The Affiliated Hospital of Liaoning University of Traditional Chinese Medicine, Inner Mongolia People’s Hospital, Hohhot First Hospital, The Second Affiliated Hospital of Baotou Medical College, The First Affiliated Hospital of Henan University of TCM, Henan Province Hospital of TCM, The First Affiliated Hospital of Henan University, Zhengzhou City Hospital of Traditional Chinese Medicine, BenQ Medical Center, The Affiliated Hospital of Jiangxi University of Chinese Medicine, Shanxi Province Hospital of TCM, Shanxi Hospital of Integrated Traditional And Western Medicine, Shaanxi Province Hospital of Chinese Medicine, The Second Teaching Hospital of Shaanxi University of TCM, The Affiliated Hospital of Shaanxi University of Chinese Medicine, Xinjiang Uygur Autonomous Region Chinese Medicine Hospital, The First Affiliated Hospital of Xinjiang Medical University, Guangdong Province Hospital of TCM, LongHua Hospital Shanghai University of Traditional Chinese Medicine, Shuguang Hospital Affiliated to Shanghai University of TCM, Shanghai East Hospital Affiliated to Tongji University, Chengdu Hospital Affiliated to Chengdu University of TCM, The First Hospital of Harbin, Panjin Center Hospital, Sanming Hospital of Integrated Traditional and Western, Wuhan Puren Hospital.
Funding
This study is supported by the Ministry of Science and Technology of the People’s Republic of China’s Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (NO. 2013BAI02B02), Ministry of Education of People’s Republic of China “Program for Innovative Research Team in University” (NO. IRT_16R54), and Tianjin Science and Technology Program (No. 15ZXLCSY00020).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study is conducted in accordance with the Declaration of Helsinki and has been approved by the ethics committee in First Teaching Hospital of Tianjin University of Traditional Chinese Medicine (Ethics approval number: TYLL2015[K] No.004).
Informed Consent
Written informed consent was obtained from all patients or their legally authorized representatives before randomization.
Rights and permissions
About this article
Cite this article
Wang, X., Zhao, Z., Hou, Y. et al. Assessment of Complementary Treatment with Yiqi Fumai Lyophilized Injection on Acute Decompensated Ischemic Heart Failure (ACT-ADIHF): Rationale and Design of a Multicenter, Randomized, Controlled Trial. Cardiovasc Drugs Ther 32, 295–300 (2018). https://doi.org/10.1007/s10557-018-6791-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-018-6791-0