Abstract
Purpose
Cholesterol efflux from macrophages to HDL, measured in vitro, is augmented by treatment with agents which raise HDL cholesterol. In vitro, cholesterol depletion by statins is known to trigger a positive feedback on the cholesterol synthetic pathway via sterol regulatory element-binding protein (SREBP) transcription and changes in expression of SREBP regulated genes including microRNA33 (miR33) which is co-transcribed with SREBP and down-regulates ABCA1 and ABCG1 expression.
Methods
We investigated whether miR33 up-regulation, associated with SREBP increased transcription by statins, reduces macrophage ATP-binding cassette (ABC) transporter expression, thereby decreasing HDL-mediated cholesterol efflux at the tissue level.
Results
In human macrophage THP-1 cells cholesterol-loaded with acetylated LDL, incubation with 1 μM atorvastatin increased miR33 by 33 % (P < 0.05), and decreased ABCA1 messenger RNA (mRNA) and ABCG1 mRNA by 47 % (P < 0.05) and 27 % (NS), respectively. In J774A.1 mouse macrophage, labelled with 3H-cholesterol, ABCA1 mRNA and ABCA1-mediated cholesterol efflux were decreased by 1 μM statin: simvastatin > pitavastatin > atorvastatin > rosuvastatin > pravastatin. HDL incubated with rhCETP and dalcetrapib increased ABCA1-mediated cholesterol efflux. However, incremental simvastatin concentrations decreased cholesterol efflux to HDL treated with rhCETP and dalcetrapib. When HDL was incubated with rhCETP, addition of dalcetrapib augmented ABCA1-mediated cholesterol efflux from J774A.1 macrophages. However, simvastatin ≥1 μM virtually eliminated any HDL-ABCA1-mediated cholesterol efflux and any augmentation of that process by dalcetrapib.
Conclusions
In vitro, statins increase miR33 expression, and decrease ABCA1 expression and cholesterol efflux from peripheral tissues; this may counteract the potential benefit of agents that raise HDL and apolipoprotein A-I in statin-treated patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The strong, inverse relationship between HDL cholesterol (HDL-C) and cardiovascular risk, mainly from populations untreated with lipid-modifying agents [1], is attributed to the role of HDL in reverse cholesterol transport (RCT) [2]. Most patients at elevated cardiovascular risk are treated with a statin and, while controversial from individual studies, large meta-analyses suggest low HDL-C still represents a cardiovascular risk factor on a background of statin treatment [3]. However, not all published data support this. The JUPITER trial compared 20 mg rosuvastatin with placebo for primary prevention of cardiovascular events in patients with elevated C-reactive protein. Median baseline HDL-C was ~51 mg/dl and did not change during the trial. HDL-C and apolipoprotein (Apo) A-I levels were strongly related to risk of major cardiovascular events in the placebo group but not in the rosuvastatin group [4]. The dal-OUTCOMES trial compared the cholesteryl ester transfer protein (CETP) modulator dalcetrapib [5] with placebo in nearly 16,000 patients with acute coronary syndrome; 97 % of whom were statin-treated with a mean baseline LDL cholesterol (LDL-C) of 76 mg/dl [6]. There was no relationship between baseline HDL-C or ApoA-1 levels and risk of major cardiovascular events for either treatment arm. Dalcetrapib increased HDL-C and ApoA-1 concentrations by 31 % and 11 %, respectively, with minimal effect on LDL-C and no effect on cardiovascular risk. Two trials have compared niacin with placebo, added to simvastatin [7, 8] (mean baseline HDL-C 35 and 44 mg/dl, respectively) and, although niacin raised HDL-C by ~15 %, there was no clinical benefit. Collectively, these observations suggest that, against a background of statin treatment, there may be no relationship of HDL-C to risk and no clinical benefit of raising HDL-C.
ATP-binding cassette (ABC) transporters mediate intracellular cholesterol transfer to HDL and are rate-limiting for RCT [9, 10]. Several cellular and plasma components are involved in the complex process of RCT and their role as well as their relative importance was reviewed by Rosenson et al. [11]. The cholesterol circulating in HDL originates almost exclusively from ABCA1 since, in the absence of ABCA1 [12] or decreased expression and function of ABCA1 [13], plasma levels of HDL-C are proportionally affected.
There are at least two potential mechanisms by which statins might reduce expression of the ABC transporters ABCA1 and ABCG1. The membrane cholesterol level is tightly regulated, and decreasing intracellular cholesterol concentration with statins increases the transcription of sterol regulatory element binding protein 2 (SREBP-2) to counteract the decrease in cholesterol and limit further elimination [14]. MicroRNA33 (miR33) is transcribed simultaneously and down-regulates the expression and decrease of cholesterol and phospholipid efflux transporters ABCA1 and/or ABCG1 expression [15]. The second mechanism involves the decrease by statin of oxysterols [16], which are ligands of liver X receptor (LXR) transcription factor regulating the expression of ABCA1 and ABCG1 [17]. These two mechanisms may concur to prevent excess removal of cholesterol from the cell membrane. They are shown schematically in Fig. 1.
Unified paradigm for cholesterol homeostasis. ABC, ATP-binding cassette transporter; HMG-CoAR, 3-hydroxy-3-methylglutaryl-CoA reductase; IDOL-1, inducible degrader of the LDL-R; LDL-R, LDL receptor; LXR, liver X receptor; miR33, microRNA33; mRNA, messenger RNA; SREBP-2, sterol regulatory element binding protein 2. Adapted from Marquart TJ et al. Proc Natl Acad Sci USA. 2010
Although the in vivo significance is not yet fully established, it should be noted that statins are known to differentially affect liver and peripheral tissue ABCA1 expression in vitro (reviewed by Sato [18]); these observations merit further investigation, especially for therapeutic interventions targeting cholesterol efflux mechanisms. Several reports have shown that miR33 regulates cholesterol efflux and HDL biogenesis by down-regulating ABCA1 and ABCG1 [19–21]; in addition Horie et al. [15] have reported both a time and dose-dependent decrease in ABCA1 protein in simvastatin treated THP-1.
Cholesterol efflux capacity of HDL in vitro is usually assessed by incubating samples with macrophage cell lines pre-loaded with labelled cholesterol, but not exposed to statin. Accordingly, if statin exposure of macrophages in vivo reduces their expression of ABC transporters and their capacity for cholesterol efflux to HDL, such an effect would not be identified using standard in vitro techniques.
We hypothesized that statins increase miR33 expression and reduce ABC transporter expression, thereby reducing the first step of RCT from peripheral tissues and possibly impairing the in vivo tissue cholesterol efflux expected from dalcetrapib-induced HDL remodeling.
Methods
In vitro Investigations
Cells
The human acute monocytic leukemia (THP-1) cell line was cultured in Roswell Park Memorial Institute (RPMI) 1640 medium, 10 % FBS, 50 μM ß-mercaptoethanol, 10 U/ml penicillin, and 10 μg/ml streptomycin. J774A.1 mouse macrophages were cultured in DMEM containing 1 g/l D-glucose, 10 % FBS, and antibiotics as above. THP-1 cells were seeded to 100,000 cells/100 μl culture medium containing 100 nM phorbol-12-myristate-13-acetate and J774A.1 cells to 40,000 cells/100 μl culture medium, in 96-well plates. Cholesterol loading was performed the next day by incubation for 24 h with 4 μCi/ml 3H-cholesterol and acetylated LDL (acLDL) (acLDL: 50 μg/ml THP-1, 25 μg/ml J774A.1). All analyses were performed in triplicate.
Expression of miR33, ABCA1, ABCG1, and LDL receptor messenger RNA, in THP-1 cells
Cholesterol-loaded THP-1 cells were incubated with atorvastatin (1 μM) or simvastatin (0.1, 1 and 10 μM) for 24 h. Gene expression analysis was performed either by quantitative polymerase chain reaction or using branched DNA technology (QuantiGene 2.0, Panomics).
Cholesterol Efflux
THP-1 cells
Cholesterol-loaded cells were washed twice with 200 μl washing buffer (phosphate buffered saline + 2 mg/mL BSA), replaced by 100 μl equilibration/efflux medium (RPMI + 2 mg/ml BSA) ± LXR/retinoid X receptor agonist (50 nM RO0721957/5 nM RO0264456) and simvastatin (0.1, 1, and 10 μM) and incubated overnight. Cells were then incubated during a 6-h efflux period with 5 μg/ml ApoA-1 (cholesterol efflux acceptor) and simvastatin (0.1, 1, and 10 μM). Following the efflux period, supernatants were filtered and cells lysed in NP40 1 %. Supernatants and cell lysates were transferred to LumaPlate-96 (Perkin Elmer) and radioactivity determined. Cholesterol efflux was expressed as percent 3H-activity in the medium relative to total 3H-activity (medium and cell lysate).
J774A.1 cells
Cholesterol-loaded cells were washed as described for THP-1 cells and incubated overnight with each of five different statins (atorvastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin) over a broad concentration range (0.0001 − 10 μM) ± 300 μM 8-(4-Chlorophenylthio)adenosine 3′,5′-cyclic monophosphate in equilibration/efflux medium (DMEM + 2 mg/ml BSA). Cells were then incubated during a 6-h efflux period with 10 μg/ml ApoA-I and statin.
Aliquots of 1.5 mg/ml HDL-C (Intracel) were incubated overnight at 4 or 37 °C with 10 μg/ml recombinant human cholesteryl ester transfer protein and 10 μM dalcetrapib. The incubated HDL, diluted to 0.5 %, provided the cholesterol efflux acceptor during the 6-h efflux period for J774A.1 cells treated as described above.
Cell Viability
Cytotoxicity was evaluated using the ApoTox-Glo Triplex Assay (Promega).
Statistical Analysis
All data are expressed as mean ± SD. Statistical analyses employed student t test unless otherwise stated.
Results
Expression of miR33, ABCA1 and ABCG1 in Statin- Treated Human Macrophage THP-1 Cells
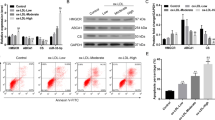
Incubation of THP-1 cells, cholesterol loaded, with 1 μM atorvastatin, was associated with a significant increase in miR33 expression (33 %, P < 0.05) and decreased ABCA1 messenger RNA (mRNA) (−47 %, P < 0.05) and ABCG1 mRNA (−27 %, NS) (Fig. 2). In non-cholesterol-loaded THP-1 cells, statin-mediated changes in mRNA expression of miR33, ABCA1, and ABCG1 (full data not shown, for ABCA1 and miR33 data see Fig. 3) were consistent with previous observations [21]. Simvastatin exposure of cholesterol-loaded THP-1 cells also produced a significant, dose-dependent decrease in ABCA1 mRNA, a slight increase in ABCG1 mRNA, and a significant increase in LDL receptor mRNA (Fig. 4).
Expression of ABCA1 and ABCG1 mRNA, and miR33, in cholesterol-loaded THP-1 cells treated with atorvastatin. *P < 0.05 vs. 0.1 % DMSO. Values are mean ± SD (triplicate). Cells incubated with acLDL for 24 h. ABC, ATP-binding cassette transporter; acLDL, acetylated LDL; DMSO, dimethyl sulfoxide; miR33, microRNA33; mRNA, messenger RNA
Effect of simvastatin (5 μM) treatment on miR33 expression in THP-1 cells. THP-1 cells were loaded or not with acLDL as described in the Methods section and treated with LXR/RXR agonist and simvastatin for 24 h. Data shown are mean ± SD, one-way ANOVA, post hoc Dunnett test. *P < 0.05; **P < 0.01 vs. control. ABC, ATP-binding cassette transporter; LXR, liver X receptor; miR33, microRNA33; RXR, retinoid X receptor
Effect of simvastatin treatment on ABCA1, ABCG1, LDL-R mRNA, and ABCA1-mediated cholesterol efflux in THP-1 cells. *P < 0.05; **P < 0.01 vs. no simvastatin. † P < 0.01 for comparison of all simvastatin concentrations combined vs no simvastatin. Values are mean + SD (triplicate). ABC, ATP-binding cassette transporter; LDL-R, LDL receptor; mRNA, messenger RNA; PPIB, peptidyl-prolyl cis-trans isomerase B
ABCA1-Mediated Cholesterol Efflux in Statin Treated THP-1 Macrophages
ABCA1-mediated cholesterol efflux from cholesterol-loaded THP-1 cells to ApoA-I was reduced by a comparable degree at all tested simvastatin concentrations, and was significant for all simvastatin concentrations combined versus no simvastatin (P < 0.01) (Fig. 4). In cholesterol-loaded J774A.1 macrophages, ABCA1 mRNA and cholesterol efflux via ABCA1 were decreased by 1 μM statin: simvastatin > pitavastatin > atorvastatin > rosuvastatin > pravastatin; i.e., the most hydrophilic exerting the smallest effects (Fig. 5); the lowest concentration of statin tested (0.0001 μM) did not differ from the no statin control. Following incubation of J774A.1 macrophages with simvastatin (0.0001 to 10 μM) and 300 μM cAMP, simvastatin significantly abrogated ABCA1-mediated cholesterol efflux, particularly at simvastatin > 0.01 μM; efflux was virtually the same as in absence of cAMP with simvastatin ≥ 1 μM (Fig. 6). This effect of simvastatin was associated with a virtually parallel significant increase in cytotoxicity.
Effect of ABCA1-mediated cholesterol efflux capacity on cytotoxicity of J774A.1 macrophages treated with simvastatin. *P < 0.05; **P < 0.01 vs. no cAMP. Values are mean ± SD, based on measures performed in triplicate. Apo, apolipoprotein; cAMP, 3′-5′-cyclic adenosine monophosphate; MFI, mean fluorescence index
We have shown previously that dalcetrapib increases the HDL remodelling activity of CETP in vitro, leading to increased pre-β1-HDL levels, the primary acceptor of cholesterol efflux via ABCA1 [5, 22]. When HDL was incubated with rhCETP, the addition of dalcetrapib increased ABCA1-mediated cholesterol efflux from J774A.1 macrophages (Fig. 7). However, incubation of the macrophages with simvastatin at concentrations ≥ 1 μM virtually eliminated any HDL-ABCA1-mediated cholesterol efflux and any augmentation of that process by dalcetrapib.
Effect of dalcetrapib on ABCA1-mediated cholesterol efflux from J774A.1 macrophages. *P < 0.05; **P < 0.01 vs. no simvastatin; †P < 0.01 for comparison of HDL + rhCETP 37 °C with vs. without dalcetrapib. Values are mean ± SD (triplicate). Cholesterol-loaded mouse J774A.1 macrophages incubated overnight and during the 6-h efflux period with 0, 0.1, 1, and 10 μM simvastatin; HDL incubated overnight at 4 or 37 °C with 10 μg/ml CETP, and 10 μM dalcetrapib was added to the cells for the 6-h efflux period. CETP, cholesteryl ester transfer protein; rh, recombinant human
Although the inhibitory effect of simvastatin on cholesterol efflux to ApoA1 is statistically significant at doses as low as 0.1 μM in THP-1 cells not loaded with cholesterol, in the presence or absence of LXR/RXR activation, a higher concentration of simvastatin (10 μM) is needed to measure a significant effect in cholesterol loaded THP-1 cells (Fig. 8) and only in the absence of LXR/RXR activation. Simvastatin did not affect efflux to mature HDL under any of the conditions tested.
Effect of simvastatin on cholesterol efflux from THP-1 cells loaded or not with cholesterol to ApoA-I vs. mature HDL particles. THP-1 cells were loaded or not with acLDL and treated with the LXR/RXR agonist and simvastatin as described in the Methods section. The cholesterol efflux acceptors ApoA-I and HDL were used at 10 μg/ml. *P < 0.05; **P < 0.01 vs. 0 μM simvastatin. acLDL, acetylated LDL; Apo, apolipoprotein; LXR, liver X receptor; RXR, retinoid X receptor
Discussion
These in vitro investigations demonstrate that statins increase miR33 expression, a suppressor of various genes including those for ABC transporters involved in RCT [20, 21]. Previous report [19] provide a putative link between statin-induced miR33 mRNA expression and reduction in both ABCA1 mRNA expression and ABCA1-mediated cholesterol efflux (Fig. 1); our results indicate that the inhibitory effect of statin (simvastatin) is specific to efflux to ApoA-I and not to mature HDL and more marked in non-cholesterol-loaded cells (Fig. 8). This is consistent with the fact that ABCA1 primarily mediates efflux to nascent HDL particles.
Our data are also consistent with previous findings that short-term treatment with low-dose atorvastatin decreased ABCA1 and ABCG1 mRNA expression in blood cells of hypercholesterolemic patients [23]. In addition, more recent studies using HDL as the lipid acceptor to evaluate ABCG1-mediated cholesterol efflux have shown that simvastatin and atorvastatin reduce cholesterol efflux in human macrophages. The marked decrease in ABCA1 protein and the potential remodelling of mature HDL to lipid-poor ApoA-I, a substrate for ABCA1, may limit interpretation of the role of ABCG1 in these studies [24]. Studies with ABCA1 and ABCG1 double knockout macrophages support a predominant role of ABCA1 in the overall efflux capacity of these cells [25].
Here, we also observed that increased HDL-induced ABCA1-mediated cholesterol efflux from J774A.1 cells by dalcetrapib was abrogated by relatively hydrophobic statins (simvastatin and atorvastatin) and, to a lesser extent, by more hydrophilic statins (pravastatin and rosuvastatin), an effect not overcome by cholesterol loading of macrophages.
Horie T. et al. [26] recently demonstrated that transgenic expression of miR33b in mice decreased the expression of ABCA1 and ABCG1 reducing HDL-C by about 35 %. In addition, macrophages from these mice showed a decreased efflux capacity to ApoA1 and HDL.
In hamsters injected with macrophages loaded with radiolabelled cholesterol we previously described an increase in plasma and fecal elimination of labelled sterol upon dalcetrapib treatment [5]. It should be noted that these experiments were performed in the absence of concomitant statin treatment. Recently, we reported [27] that dalcetrapib treatment of hamsters increased the intestinal absorption of the antioxidants lutein and zeaxanthin via an HDL pathway, which is ABCA1 and ApoA1 dependent. Simvastatin treatment decreased the expression of intestinal ABCA1, decreased lutein and zeaxanthin concentrations in plasma and liver, and abrogated the effect of dalcetrapib on lutein and zeaxanthin absorption. These data support the potential in vivo relevance of the in vitro observations reported here.
Interestingly, reported pro-diabetogenic effects of statins might be explained by a mechanism involving a decrease in ABCA1 expression, since ABCA1 plays a critical role in pancreatic insulin-secreting beta-cell physiology [28], while decrease in ABCA1 function may explain the observed impaired glycemic control and insulin secretion, without affecting insulin sensitivity in type 2 diabetic patients treated with statins [29]. In addition, miR33 has also been shown to modulate ABCA1 expression, cholesterol accumulation, and insulin secretion in pancreatic islets [30].
It should be noted that, although statins decrease ABCA1 expression in macrophages and intestine, these effects appear to be tissue-specific: statins appear to have no effect, or even to increase, expression of ABCA1 in liver (reviewed by Cerda et al. [31]). If the effect of statin treatment on HDL-C plasma level is predominantly influenced by effects on hepatic ABCA1 expression, this may explain the clinical finding that statin treatment does not reduce, and may even increase, plasma HDL-C, despite present observations indicating reduced macrophage ABCA1 expression. This hypothesis also suggests that, in statin-treated patients, the cholesterol carried by HDL may originate more from the liver than from peripheral tissues, thus explaining a less predictive and protective value of the HDL-C observed in dal-OUTCOMES.
In addition to therapeutic interventions, miR-33 expression can be upregulated in THP-1 macrophages by inflammatory conditions leading to a decrease in ABCA1 and ABCG1 expression and cholesterol efflux as very recently demonstrated by Mao et al. [32]. Thus, one may speculate that, in addition to statin treatment, the inflammatory status of Acute Coronary Syndrome patients may affect the response to HDL-raising agents.
Collectively, our observations may help reconcile the lack of clinical benefit from HDL-raising agents in statin-treated patients, with efficacy demonstrated in the absence of statin in vitro towards increasing cholesterol efflux capacity. New approaches towards HDL-raising, including a miR33 antagonist (antagomir-33), reconstituted HDL, and ApoA-I mimetics are in advanced clinical development, but to optimize their use it will be important to characterize any pharmacodynamic interaction with statin-triggered feedback on the SREBP pathways.
References
Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15.
Oram JF, Lawn RM, Garvin MR, Wade DP. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J Biol Chem. 2000;275:34508–11.
Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78.
Ridker PM, Genest J, Boekholdt SM, Libby P, Gotto AM, Nordestgaard BG, et al. HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy: an analysis from the JUPITER trial. Lancet. 2010;376:333–9.
Niesor EJ, Magg C, Ogawa N, Okamoto H, von der Mark E, Matile H, et al. Modulating cholesteryl ester transfer protein activity maintains efficient pre-beta-HDL formation and increases reverse cholesterol transport. J Lipid Res. 2010;51:3443–54.
Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99.
Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67.
HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013, 34:1279–1291.
Attie AD, Kastelein JP, Hayden MR. Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J Lipid Res. 2001;42:1717–26.
Bochem AE, van Wijk DF, Holleboom AG, Duivenvoorden R, Motazacker MM, Dallinga-Thie GM, et al. ABCA1 mutation carriers with low high-density lipoprotein cholesterol are characterized by a larger atherosclerotic burden. Eur Heart J. 2013;34:286–91.
Rosenson RS, Brewer Jr HB, Davidson WS, Fayad ZA, Fuster V, Goldstein J, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–19.
Altilia S, Pisciotta L, Garuti R, Tarugi P, Cantafora A, Calabresi L, et al. Abnormal splicing of ABCA1 pre-mRNA in Tangier disease due to a IVS2 + 5G>C mutation in ABCA1 gene. J Lipid Res. 2003;44:254–64.
Wellington CL, Yang YZ, Zhou S, Clee SM, Tan B, Hirano K, et al. Truncation mutations in ABCA1 suppress normal upregulation of full-length ABCA1 by 9-cis-retinoic acid and 22-R-hydroxycholesterol. J Lipid Res. 2002;43:1939–49.
Goldstein JL, Bose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46.
Horie T, Ono K, Horiguchi M, et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc Natl Acad Sci U S A. 2010;107:17321–6.
Wong J, Quinn CM, Brown AJ. SREBP-2 positively regulates transcription of the cholesterol efflux gene, ABCA1, by generating oxysterol ligands for LXR. Biochem J. 2006;400:485–91.
Venkateswaran A, Laffitte BA, Joseph SB, et al. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci U S A. 2000;97:12097–102.
Sato R. Sterol metabolism and SREBP activation. Arch Biochem Biophys. 2010;501:177–81.
Marquart TJ, Allen RM, Ory DS. Baldan A: miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci U S A. 2010;107:12228–32.
Najafi-Shoushtari SH, Kristo F, Li Y, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–9.
Rayner KJ, Suarez Y, Davalos A, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–3.
Maugeais C, Perez A, von der Mark E, Magg C, Pflieger P, Niesor EJ. Evidence for a role of CETP in HDL remodeling and cholesterol efflux: role of cysteine 13 of CETP. Biochim Biophys Acta. 1831;1831:1644–50.
Genvigir FDV, Rodrigues AC, Cerda A, et al. Effects of lipid-lowering drugs on reverse cholesterol transport gene expressions in peripheral blood mononuclear and HepG2 cells. Pharmacogenomics. 2010;11:1235–46.
Wang W, Song W, Wang Y, Chen L, Yan X. HMG-CoA reductase inhibitors, simvastatin and atorvastatin, downregulate ABCG1-mediated cholesterol efflux in human macrophages. J Cardiovasc Pharmacol. 2013;62:90–8.
Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, et al. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–47.
Horie T, Nishino T, Baba O, Kuwabara Y, Nakao T, Nishiga M, et al. MicroRNA-33b knock-in mice for an intron of sterol regulatory element-binding factor 1 (Srebf1) exhibit reduced HDL-C in vivo. Sci Rep. 2014;4:5312.
Niesor EJ, Chaput E, Mary JL, Staempfli A, Topp A, Stauffer A, et al. Effect of compounds affecting ABCA1 expression and CETP activity on the HDL pathway involved in intestinal absorption of lutein and zeaxanthin. Lipids. 2014;49:1233–43.
Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, et al. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med. 2007;13:340–7.
Bellia A, Rizza S, Lombardo MF, Donadel G, Fabiano R, Andreadi K, et al. Deterioration of glucose homeostasis in type 2 diabetic patients one year after beginning of statins therapy. Atherosclerosis. 2012;223:197–203.
Wijesekara N, Zhang LH, Kang MH, Abraham T, Bhattacharjee A, Warnock GL, et al. Hayden MR: miR-33a modulates ABCA1 expression, cholesterol accumulation, and insulin secretion in pancreatic islets. Diabetes. 2012;61:653–8.
Cerda A, Hirata MH, Hirata RDC. Molecular mechanisms underlying statin effects on genes involved in the reverse cholesterol transport. Drug Metabol Drug Interact. 2012;27:101–11.
Mao M, Lei H, Liu Q, Chen Y, Zhao L, Li Q, et al. Effects of miR-33a-5P on ABCA1/G1-mediated cholesterol efflux under inflammatory stress in THP-1 macrophages. PLoS One. 2014;9:e109722.
Acknowledgments
The authors thank Dr Teresa Haigh for her critical review and valuable contribution to this paper. Editorial assistance was provided by Prime Healthcare during the preparation of this report and funded by F Hoffmann-La Roche Ltd. All conclusions and opinions expressed are those of the authors. The current address of Dr Kallend is The Medicines Company, Zürich, Switzerland.
Sources of Funding
This study was funded by F. Hoffmann-La Roche Ltd.
Disclosures
EJN, AP, AD, GS, RB, and MA are employees of F. Hoffmann-La Roche Ltd. DK was an employee of F. Hoffmann-La Roche Ltd at the time the study was performed. GGS, through his institution, has received research grant support from Roche.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niesor, E.J., Schwartz, G.G., Perez, A. et al. Statin-Induced Decrease in ATP-Binding Cassette Transporter A1 Expression via microRNA33 Induction may Counteract Cholesterol Efflux to High-Density Lipoprotein. Cardiovasc Drugs Ther 29, 7–14 (2015). https://doi.org/10.1007/s10557-015-6570-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-015-6570-0