Corrosion resistance of equipment for potash production by a halurgical method is studied. Results are provided for accelerated electrochemical corrosion resistance tests for stainless steels in model working production media.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The problem of equipment corrosion in halurgical production of potash has existed from the time of generation of this industry. In 1927 in a bulletin about the development of the potassium industry in Germany and France Rice and Davis [1] provided data about a requirement for replacing equipment for dissolving ore every three years. Loss due to corrosion (in equipment depreciation) comprised up to one third of the final product’s cost. In a handbook [2], there are data about a requirement for performing major overhaul of equipment after each 8460 h of operation with replacement of the main parts of equipment, and this ultimately leads to an increase in potash cost. Thus, the task of improving corrosion resistance of equipment in potash production remains important.
The production process for preparing potash by a halurgical method consists of extracting soluble substance from solid material (sylvinite ore) by means of a solvent (mother liquor). The method is based on combined dissolution of potassium chloride (KCl) and sodium chloride (NaCl) in water at different temperatures. During cooling of saturated solution, KCl crystallizes from it.
In halurgical production of potash, a three-stage direct leaching scheme is used. Hot leaching of potassium chloride is performed in two stages with recuperation of halite tailings heat in three stages. The equipment operates by a direct flow scheme and is installed successively. Sylvinite ore is charged into the first equipment, within which there is heating to the mother liquor dissolution temperature. After this, the ore is fed into the second equipment for final dissolution by liquor, whose temperature exceeds 100°C. The residue from the second equipment is loaded by an elevator with a screw dissolver, intended for recuperating halite tailings heat.
Working media contain chloride salts and solid phase, causing pitting corrosion and corrosive-erosive wear, respectively (Table 1). The high corrosion and erosion activity of working media is strengthened at high temperature.
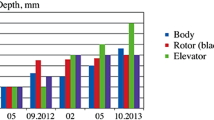
Examination of equipment shows that significant corrosive and erosive wear occurs in pipelines, equipment, settling tanks for hot liquor, pumps, elevator buckets, etc. Various coatings are used for protection: dissolution equipment is covered with diabase paste; equipment and settling tanks are rubberized or lined with Antegmit plates; drainage troughs, pans, casings for heat insulation are made from polymer materials and protected by pastes based on asbovinyl; metal pipelines are protected by porcelain linings based on special cements or rubberized [3]. The bottom of equipment is covered with steel plates, and walls of equipment and stirrers are covered with diabase paste. The body of the thickener and tanks filled with hot solutions are lined with plates made from diabase and Antegmit. The body of the vacuum crystallizer is lined with rubber sheet [4].
In order to manufacture assemblies and components in contact with working medium, steel of grade 08Kh21N6M2T is used, most resistant in this medium (compared with steels of grades 12Kh18N10T and 10Kh17N13M2T) and recommended by organizations operating equipment and specialized institutes such as VNII Galurgii and UkrNIIkhimimmash.
Recently with the aim of reducing the cost some producers have begun to use steel 1.4571 and its direct analogs. This steel was used to manufacture and put into operation the chain of a hopper and rotor, although operating experience showed that this steel did not exhibit corrosion resistance in the working medium.
In order to manufacture equipment for potash production ferrite-austenite alloys are interesting, in particular steel 1.4410 (Table 2), whose erosion resistance and pitting index are several times higher than that of austenitic steels. These alloys are easily welded and the thermal expansion coefficient is close to that of carbon steels [5].
With the aim of comparative evaluation of the corrosion resistance of steels 08Kh21N6M2T and 1.4410, accelerated electrochemical tests were carried out at 70 ± 2°C in model solution, %: NaCl – 17, KCl – 13; in the working solution of production line B with the average chemical composition, %: KCl – 13.83, NaCl – 17.50, MgCl – 1.79, CaSO4 – 0.19, CaCl2 – 1.77.
Electrochemical studies of corrosion resistance of specimens were performed using a P-5827M potentiostat in a YaSE-2 three-electrode electrochemical cell without separation of the anodic and cathodic spaces. The reference electrode was saturated silver chloride, and the subsidiary electrode was platinum. Anodic potentiodynamic curves were recorded with a potential sweep rate of 1.8 V/h. Preparation of steel specimens for electrochemical study and accelerated electrochemical testing was performed in accordance with GOST 9.912–89 [6].
In the model solution, steels 08Kh21N6M2T and 1.4410 are in a passive condition (Fig. 1). However, the pitting repassivation potential for steel 08Kh21N6M2T is 40 mV more positive than the free corrosion potential, and this can lead to the occurrence of pitting with insignificant changes in conditions for carrying out the process. The region of stable passivation for steel 1.4410 is somewhat greater (100 mV).
In a production working solution, steel 08Kh21N6M2T is subject to pitting corrosion in a steady state (Fig. 2); steel 1.4410 is in a passive condition, and the passivation potential is more positive by 100 mV than the corrosion potential for free corrosion.
Corrosion tests for steels 08Kh21N6M2T and 1.4410 carried out by a gravimetric method in a working solution under laboratory conditions showed that after 300 h of testing the corrosion rate did not exceed 0.005 g/(m2·h) and 0.001 g/(m2·h), respectively.
The corrosion rate for the test steel in the model solution is higher than in a working production medium. This may be explained by the presence in the production medium of sulfate ions, which as is well known exhibit inhibiting properties. However, taking account of the fact that under actual operating conditions a production medium contains solid phase, pro-moting erosive removal of a passive film, it may be predicted that the corrosion resistance of steel under operating conditions will be higher [7–10].
In order to determine the possible passivating effect of mother liquor on steels 08Kh21N6M2T and 1.4410, test specimens were held in mother liquor solution for 24, 36, and 72 h followed by recording of potentiodynamic dependences in working solution (Fig. 3).
Research shows that holding of specimens of 08Kh21N6M2T and 1.4410 steels in mother liquor not only does not give rise to passivation, but in contrast facilitates pitting formation.
Thus, this research confirms the high corrosive activity of production media for potash halurgical manufacture, and therefore it is necessary to use more corrosion resistant alloys or coatings. Another method for reducing corrosion losses is replacement of the halurgical manufacturing method by flotation and combined methods for potash preparation.
References
G. S. Rice and J. A. Davis, Potash Mining in Germany and France, Bulletin 274, U.S. Department of Commerce, Bureau of Mines, Washington (1927).
G. F. Kiselev,V. I. Kolpachkov, and A. I. Yashura, System of Technical Servicing and Repair of Production Equipment for Mineral Fertilizer Production Enterprises, Khimiya, Moscow (1991).
Recommendations for Design of Corrosion Protection for Mineral Fertilizer Storage Structures, Stroizdat, Moscow (1983).
E. Ya. Mel’nikov, V. P. Saltanova, A. M. Maumova, and Zh. S. Blinova, Inorganic Substance and Mineral Fertilizer Technology, Khimiya, Moscow (1983).
H. S. Khatak and R. Baldev, Corrosion of Austenitic Stainless Steels. Mechanism, Mitigation and Monitoring, Cambridge, England (2002).
GOST 9.912–89, Single Protection System from Corrosion and Ageing. Corrosion Resistant Steels and Alloys. Accelerated Test Methods for Pitting Corrosion Resistance.
K. R. Tarantseva and V. S. Pakhomov, Protec. Metals Phys. Chem, Surf., 46, No. 3, 359 (2010).
K. R. Tarantseva, Protec. Metals Phys. Chem, Surf., 46, No. 1, 139 (2010).
K. R. Tarantseva and V. S. Pakhomov, Chem. Petrol. Eng., 45, No. 5–6, 381 (2009).
K. Tarantseva and V. Pakhomov, NACE – Int. Corros. Conf. Ser. 2, 1084 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimicheskoe i Neftegazovoe Mashinostroenie, No. 1, pp. 34–36, January, 2015.
Rights and permissions
About this article
Cite this article
Tarantseva, K.R., Firsova, O.V. & Palicheva, A.S. Study of Equipment Corrosion Resistance in the Production of Potash by a Halurgical Method. Chem Petrol Eng 51, 49–53 (2015). https://doi.org/10.1007/s10556-015-9997-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10556-015-9997-z