Abstract
Cardiac Magnetic Resonance (CMR) is increasingly being used for diagnosing various cardiac conditions. Parametric mapping enables quantitative myocardial characterization by directly measuring myocardial T1 and T2 values. However, reference values of parametric mapping are not standardized across different vendors and scanners, causing drawbacks for clinical implementation of this technique across different sites. We assessed the reference ranges of native T1 and T2 values in a healthy Maltese cohort to establish a local parametric mapping service. Healthy subjects [n = 51; mean age 36.0 (range 19–59) years] with normal cardiac function on CMR were recruited. Subjects underwent uniform parametric mapping pulse sequences [MOLLI 5b(3b)3b for native T1 mapping, and gradient echo single shot FLASH readout for T2 mapping] on a 3 T Siemens MAGNETOM Vida scanner. Native T1 and T2 values were measured by placing a region of interest within the interventricular septum at midventricular level. Intra- and inter-observer variability were assessed using Bland–Altman plots. Mean ± 1.96 SD was used as a reference range. Mean native T1 and T2 values were 1200.1 ± 30.7 ms and 39.5 ± 1.8 ms, respectively. There was no significant bias in repeated measurements by the same and different observers. For the first time in Malta, we established the native T1 and T2 parametric mapping reference values for healthy Caucasian Maltese individuals. This will assist cardiologists to establish diagnosis, disease progression, and response to treatment of various myocardial diseases locally.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiac magnetic resonance (CMR) is increasingly being used for the diagnosis of various cardiac conditions, including ischemic heart disease, cardiomyopathy, valvular heart disease, and congenital heart disease. This non-invasive diagnostic tool provides reliable measurement of cardiac function, structure, tissue characterization, and metabolism. [1]
More recently, the introduction of CMR parametric mapping has enabled quantitative myocardial characterization by directly measuring the values of myocardial T1, T2, and extracellular volume (ECV) [2]. Native T1 and T2 values are especially useful for the evaluation of a range of myocardial diseases, including, but not limited to, cardiomyopathy, myocarditis, and ischemic heart disease.
The current method used for assessing myocardial fibrosis by late gadolinium enhancement has some limitations. In late gadolinium enhancement, image contrast relies on the difference in signal intensity between fibrotic and “normal” myocardium, and therefore such differences may not exist if the disease process is diffuse [3]. Furthermore, there is no clear consensus on the intensity threshold settings to use for clinical assessment of myocardial fibrosis on late gadolinium enhancement [3]. In contrast, parametric mapping can directly and objectively provide pixel-by-pixel magnetic resonance relaxometric values.
In 2017, the Society for Cardiovascular Magnetic Resonance (SCMR) published clinical recommendations for CMR parametric mapping [4]. Values of T1 and T2 are not only affected by intrinsic tissue characteristics, but also depend on the employed pulse sequence, hardware, and post-processing technqiue [4]. As a consequence, native parameters should only be compared to other parameter values if they are obtained using the same scanner and acquired under the same conditions [4]. Hence, setting a local reference range is strongly advised for clinical use of myocardial mapping.
The aim of this study was to establish local reference ranges for native T1 and T2 in Caucasian Maltese population.
Methods
Study population
“Healthy subjects”, defined as individuals without significant past medical history, no reported symptoms, and normal cardiac function on cine CMR were included. Subjects were strictly not awaiting CMR for any clinical reason and had to complete a questionnaire prior to CMR to confirm that they were free of any known heart disease, hypertension, or overt symptoms of heart disease. Subjects provided informed consent prior to their participation in the study. The study was performed at the Department of Radiology (Mater Dei Hospital, Malta).
CMR Acquisition parameters
Native T1 mapping sequence and image protocol were performed using a 3 T Siemens MAGNETOM Vida scanner with software version Syngo MR XA20 and 18-channel body coil. Subjects underwent an imaging protocol consisting of initial scout acquisitions followed by long axis cine to assess cardiac size and function, native T1 mapping sequence, and T2 mapping sequence. Both native T1 and T2 sequences were performed using 3 visually-planned, short-axis slices (base, mid-ventricular, and apex).
Modified look-locker inversion recovery (MOLLI) with 11 heartbeats 5(3)3 scheme and balanced Steady State Free Precision (bSSFP) readouts were used for native T1 mapping with a flip angle of 35 degrees, TE of 0.97 ms, and TR of 2.32 ms. T2 mapping was performed with gradient echo single shot FLASH readout with multiple T2 preparations and recovery period. Due to ethical reasons, we did not administer contrast; hence, only native mapping was performed.
Image analysis
All CMR images were anonymized, and analysis was performed using commercially available software (Syngo.Via Client 5.2).
The native T1 and T2 values were measured by placing a region of interest (ROI) conservatively within the interventricular septum at midventricular level after a careful evaluation of image quality. In case of compromised image quality at midventricular level, the basal septum was used for the assessment. The ROI was carefully placed to avoid contamination from the blood pool.
On two separate days, the same observer (KY) repeated the measurements for intra-observer variation. The measurements were repeated for a total of three times to obtain an average value of 3 separate drawn ROIs for the final analysis. In addition, to assess inter-observer variability, an independent observer (CP) measured native T1 and T2 values on the same set of images.
Statistical analysis
Statistical analysis was performed using SPSS statistical software (IBM SPSS Statistics 23; Chicago, IL, USA).
Kolmogorov–Smirnov test was used to assess for normal distribution of data. Tukey robust approach was performed to identify and remove outliers. Mean ± 1.96 SD was used as a reference range. No individuals had T1 and T2 values outside of the reference range. Comparisons of reference ranges between sexes were presented as described by Higgins et al. [5] Bland–Altman plots were generated in Microsoft Excel 2013 to assess both intra- and inter-observer variability.
Results
Fifty-one healthy subjects (24 males and 27 females) with a mean age of 36.0 years (range 19–59 years) were recruited. All participants were Caucasian Maltese nationals. Characteristics of healthy subjects are shown in Table 1.
All CMR scans were successfully completed using the full study protocol and without any adverse events. Image quality was sufficient for analysis for all scans performed. Statistical analyses were conducted as outlined previously. The Kolmogorov–Smirnov test for normality resulted in p values of 0.773 and 0.125 for native T1 and T2, respectively (in this case, p > 0.05 signified that the null hypothesis of normal distribution was not rejected i.e., accepting that data followed normal distribution).
Reference values of native T1 and T2 CMR parameters of the 51 subjects are shown in Table 2. Mean native T1 value was 1200.1 ± 30.7 ms, with a reference range of 1139.9 ms to 1260.4 ms. The mean T2 value was 39.5 ± 1.8 ms, with a reference range of 35.9 ms to 43.0 ms. An example of native T1 and T2 mapping in a healthy volunteer is presented in Fig. 1
Sex-related differences of native T1 and T2 values are summarized in Table 3.
There was no significant bias in repeated measurements by the same and different observers (except for a tendency for the same observer to derive lower T2 on the second measurement, albeit by a clinically insignificant amount). Limits of agreement were within ± 35.0 ms and ± 1.6 ms for repeated T1 and T2 measurements, respectively. (Fig. 2 and Fig. 3).
Discussion
This study analyzed the native T1 and T2 values of a population of Maltese healthy individuals using the 3 T Siemens Vida scanner at Mater Dei Hospital, Malta. The aim of the study was to establish parametric mapping service in Malta. This is the first study on native T1 and T2 reference values for Caucasian Maltese population.
As reported previously, several factors influence T1/T2 reference values, including scanner vendor, strength of magnetic field, age, and gender. Due to these factors, the SCMR guidelines recommend the use of local reference ranges for native T1 and T2 mapping; if local reference ranges are not available, quantitative results should not be reported clinically. [4]
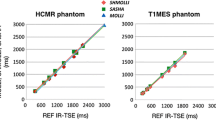
Several studies have been conducted to establish native T1 and T2 values in various populations of healthy volunteers (Table 4). Native T1 and T2 values were compared across studies (Fig. 4 and Fig. 5).
The native T1 and T2 values established in this study correlate closely with those by Pons-Lladó (1230 ± 38.5 ms for native T1 and 39 ± 2.2 ms for T2) who used the exact same vendor and type of scanner (Siemens MAGNETOM Vida), magnetic field, and sequence as the present study [6]. Native T1 values in the present study were also similar to those by Dong et al. (1202 ± 45 ms) and Teixeira et al. (1207.9 ± 18.2 ms for native T1) who used the same vendor (Siemens), magnetic field, and pulse sequence [7, 8]. Nonetheless, minor differences in native T1 and T2 values were seen between the present study and Lee et al. (1266.03 ± 32.86 ms for native T1 and 40.09 ± 2.45 ms for T2) [9]. The dissimilarity in native T1 values between the studies by Pons-Llado, Teixeira et al., Dong et al., Lee et al., and the present study with those reported by Roy et al. (1162 ± 81 ms) [10], and Tribuna et al. (1124.9 ± 55.2 ms) [11] might be explained by the variance in scanner vendor. Furthermore, 73% of participants in the study by Roy et al. had at least one cardiovascular risk factor.
Flip angle is also known to influence native T1 measurement accuracy. A high MOLLI sequence flip angle is typically associated with underestimation of native T1 values while it increases signal-to-noise ratio (SNR), leading to higher precision. [12]
With regards to T2 values, the variance between our values and T2 values seen in Roy et al. (52 ± 7.0 ms) [10] and Grantiz et al. (51.6 ± 3.0 ms) [13] may be explained by the variance in vendor and pulse sequence utilized in these studies, as previously described by Baeßler et al. [14] Of note, there is a clinical relevant inter-scanner variability in T2 values, which causes major drawbacks for the clinical implementation of this technique across different sites [14]. Additionally, mean T2 times vary significantly according to different magnetic field strengths.
Myocardial native T1 and T2 mapping is useful in detecting a range of myocardial diseases such as myocarditis, infiltrative myocardial disease (including Anderson-Fabry disease and cardiac amyloidosis), as well as for assessing the etiology of heart failure. Furthermore, this technique can assist clinicians in assessing disease progression and response to treatment. Therefore, standardization of parametric values across different vendors and scanners is imperative. A strong collaboration between vendors may help in standardizing reference values for parametric mapping.
Conclusion
For the first time in Malta, we established the native T1 and T2 parametric mapping reference values for healthy Maltese individuals using the Siemens MAGNETOM Vida scanner. This will further assist cardiologists to establish diagnosis, disease progression, and response to treatment of various myocardial diseases. Due to the high variability in values between different scanners and vendors, standardization across vendors is warranted.
Data availability
Above listed authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.This work has not been presented elsewhere.
References
Situ Y, Birch SCM, Moreyra C, Holloway CJ (2020) Cardiovascular magnetic resonance imaging for structural heart disease. Cardiovasc Diagn Ther 10(2):361–375. https://doi.org/10.21037/cdt.2019.06.02
Ferreira VM, Piechnik SK (2020) CMR parametric mapping as a tool for myocardial tissue characterization. Korean Circ J 50(8):658–676. https://doi.org/10.4070/kcj.2020.0157
Mewton N, Liu CY, Croisille P, Bluemke D, Lima JAC (2011) Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 57(8):891–903. https://doi.org/10.1016/j.jacc.2010.11.013
Messroghli DR, Moon JC, Ferreira VM et al (2017) Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imagi. J Cardiovasc Magn Reson 19(1):75. https://doi.org/10.1186/s12968-017-0389-8
Higgins DM, Keeble C, Juli C, Dawson DK, Waterton JC (2019) Reference range determination for imaging biomarkers: myocardial T(1). J Magn Reson Imaging 50(3):771–778. https://doi.org/10.1002/jmri.26683
Pons-Lladó G (2019) Reference normal values for myocardial T1 and T2 maps with the MAGNETOM Vida 3T system and case examples from clinical practice. MAGNETOM Flash 72:29–33
Teixeira T, Hafyane T, Stikov N, Akdeniz C, Greiser A, Friedrich MG (2016) Comparison of different cardiovascular magnetic resonance sequences for native myocardial T1 mapping at 3T. J Cardiovasc Magn Reson 18(1):65. https://doi.org/10.1186/s12968-016-0286-6
Dong Y, Yang D, Han Y et al (2018) Age and gender impact the measurement of myocardial interstitial fibrosis in a healthy adult chinese population: a cardiac magnetic resonance study. Front Physiol. https://doi.org/10.3389/fphys.2018.00140
Lee E, Kim PK, Choi BW, Jung JI (2020) Phantom-validated reference values of myocardial mapping and extracellular volume at 3T in healthy koreans. Investig Magn Reson Imaging 24(3):141–153. https://doi.org/10.13104/imri.2020.24.3.141
Roy C, Slimani A, de Meester C et al (2017) Age and sex corrected normal reference values of T1, T2 T2* and ECV in healthy subjects at 3T CMR. J Cardiovasc Magn Reson 19(1):72. https://doi.org/10.1186/s12968-017-0371-5
Tribuna L, Oliveira PB, Iruela A, Marques J, Santos P, Teixeira T (2021) Reference values of native T1 at 3T cardiac magnetic resonance-standardization considerations between different vendors. Diagnostics. https://doi.org/10.3390/diagnostics11122334
Kellman P, Hansen MS (2014) T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson 16(1):2. https://doi.org/10.1186/1532-429X-16-2
Granitz M, Motloch LJ, Granitz C et al (2019) Comparison of native myocardial T1 and T2 mapping at 1.5T and 3T in healthy volunteers. Wien Klin Wochenschr 131(7):143–155. https://doi.org/10.1007/s00508-018-1411-3
Baeßler B, Schaarschmidt F, Stehning C, Schnackenburg B, Maintz D, Bunck AC (2015) A systematic evaluation of three different cardiac T2-mapping sequences at 1.5 and 3T in healthy volunteers. Eur J Radiol 84(11):2161–2170. https://doi.org/10.1016/j.ejrad.2015.08.002
Funding
The authors received no financial support for the research authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
K.Y. and L.M.Y. organized a whole study, wrote the main manuscript text and prepared figures and tables. M.A. and A.B. contributed in statistical analysis and reviewed the main manuscript text. C.P.M. and L.M. collected data. L.R. helped recruiting subjects.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamagata, K., Yamagata, L.M., Abela, M. et al. Native T1 and T2 reference values for maltese healthy cohort. Int J Cardiovasc Imaging 39, 153–159 (2023). https://doi.org/10.1007/s10554-022-02709-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-022-02709-6