Abstract
We aimed to investigate left atrial (LA) myocardial dynamics during reservoir phase using three-dimensional speckle-tracking echocardiography (3DSTE) focusing on its longitudinal-circumferential relationship in patients with left ventricular (LV) hypertrophy and clarifying the difference in LA myocardial reservoir dynamics between hypertrophic cardiomyopathy (HCM) and hypertension with LV hypertrophy (HT-LVH). We studied 4 age-matched groups consisting of 27 patients with HCM, 16 with HT-LVH, 22 hypertensive patients without LV hypertrophy (HT), and 18 normal controls. Using 3DSTE, we measured LA global longitudinal strain (LA-LSR), global circumferential strain (LA-CSR), and global area strain (LA-ASR) during the reservoir phase, as well as LV global longitudinal strain (LV-LS), global circumferential strain (LV-CS), and global area strain (LV-AS). LA-LSR was significantly lower in the HCM and HT-LVH groups than in the controls, but there was no significant difference between the HCM and HT-LVH groups. LA-CSR and LA-ASR were significantly lower in the HCM group than in the other three groups, among which no significant difference was detected. In all subjects, LA-LSR was significantly correlated with LV-LS but not with LV-CS. LA-CSR was correlated with neither LV-LS nor LV-CS. In conclusion, both longitudinal and circumferential LA myocardial expansion during reservoir phase were reduced in HCM, while only the longitudinal one was reduced in HT-LVH. Reduction of LA circumferential expansion may reflect a more serious and intrinsic impairment of LA myocardial distensibility in HCM. Measuring LA-CSR and LA-ASR using 3DSTE would contribute to a more accurate understanding of LA reservoir function abnormality in HCM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Impairment of left atrial (LA) reservoir function is known to be present in patients with hypertrophic cardiomyopathy (HCM) based on several clinical studies using LA volumetric approaches such as cineangiogram [1], conventional echocardiography [2], and cardiac magnetic resonance imaging (CMR) [3]. Subsequently, with the technological advances in noninvasive myocardial strain analysis using echocardiography or CMR, a decrease in LA myocardial expansion during the reservoir phase was detected in patients with HCM [4,5,6,7] and also in those with hypertension [8, 9]. In those studies, however, LA myocardial dynamics was analyzed only in the cardiac longitudinal direction.
More recently, three-dimensional (3D) speckle tracking echocardiography (3DSTE) enabled the quantitation of three-dimensional myocardial deformation and was applied in a few studies to the analysis of LA myocardial dynamics in patients with left ventricular (LV) hypertrophy [10, 11]. However, there has been no consensus on the longitudinal-circumferential relationship of LA myocardial dynamics during the reservoir phase or on the difference between HCM and hypertensive heart. Thus, this study aimed to investigate LA myocardial dynamics during the reservoir phase using 3DSTE focusing on its longitudinal-circumferential relationship in patients with LV hypertrophy, and to examine the difference in three-dimensional LA myocardial dynamics during the reservoir phase between patients with HCM and those with hypertensive LV hypertrophy.
Subjects and methods
Subjects
We retrospectively examined 31 patients with hypertrophic cardiomyopathy (HCM), 21 age-matched patients with hypertension with LV hypertrophy (HT-LVH), 27 age-matched hypertensive patients without LV hypertrophy (HT), and 22 age-matched normal control subjects who underwent echocardiographic examination using an Artida ultrasonographic system and data acquisition for LA strain analysis using 3DSTE in our laboratory between January 2016 and May 2019. They did not have atrial fibrillation, atrial septal aneurysm, moderate or severe mitral regurgitation, significant mitral annular calcification, LV systolic dysfunction (LV ejection fraction < 50%), or hemodialysis. Among the initial subjects, 3DSTE analysis for LA was not successful due to inadequate echocardiographic image quality in 18 subjects. The remaining 83 subjects (27 patients with HCM, 16 with HT-LVH, 22 with HT, and 18 normal controls) were included in the present study.
HCM was defined according to the current European Society of Cardiology guidelines [12]. Among our 27 HCM patients, asymmetric septal hypertrophy was found in 19 (70%) and apical hypertrophy in 8 (30%). LV outflow tract obstruction at rest was present in 6 (22%). A diagnosis of HT was made when blood pressure measurements after sufficient physical and mental rest on 2 or more subsequent visits were consistently > 140 mmHg systolic or > 90 mmHg diastolic, or when a patient with a history of hypertension was receiving antihypertensive medications. We defined HT-LVH as both hypertension and LV hypertrophy (LV mass index > 115 g/m2 for males, > 95 g/m2 for females and/or relative wall thickness > 0.42). The control group consisted of age-matched patients who underwent an echocardiographic examination in our laboratory and did not have any echocardiographic abnormalities or any history of cardiac diseases.
Conventional echocardiography
Using an Artida ultrasonographic system (Canon Medical Systems, Otawara, Japan) equipped with a PST-30BT transducer, a standard echocardiographic examination was performed in every patient according to the guidelines of the American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) [13]. In patients with HCM, the greatest wall thickness among 16 LV segments at end-diastole was measured as the LV maximum wall thickness. In the other subject groups, the thicknesses of interventricular septum and LV posterior wall were measured in the end-diastolic parasternal short-axis image at the chordal level, and the greater of the two thicknesses was defined as the LV maximum wall thickness. Left atrial volume index (LAVI) was measured from apical two-chamber and four-chamber images using the biplane disk-summation method. LV end-diastolic and end-systolic volumes were also measured using the biplane disk-summation method, and the LV ejection fraction (LVEF) was calculated.
Pulsed Doppler echocardiography was performed to measure peak early diastolic and atrial systolic transmitral flow velocities (E and A, respectively), and the E/A ratio was calculated. Tissue Doppler imaging of the mitral annulus was performed in the apical four-chamber view to measure peak early diastolic annular velocity (e′) was measured at the septal and lateral side and then averaged. The E/e′ ratio was calculated using averaged e′ value. LV diastolic dysfunction grade was assessed according to the current ASE/EACVI recommendations [14].
Three-dimensional speckle-tracking echocardiography
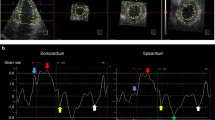
Using the Artida system equipped with a PST-25SX matrix-array transducer, 3DSTE was performed immediately following the conventional echocardiographic study. Within a breath-hold and during a constant RR interval, an apical full-volume image of LA and one of LV were acquired. In all subjects, 3D data were collected through six consecutive cardiac cycles. To improve the temporal and spatial resolution of each of the LA and LV volumetric images, the depth and sector width were decreased as much as possible. The mean volume rate of LA images was 33.5 ± 4.9 (16–40) vps and that of the LV images was 33.9 ± 5.3 (16–40) vps. All the 3D data sets were stored for offline analysis and exported to a workstation (UltraExtend Advanced Cardiology Package version 2.7, Canon Medical Systems). We set several markers on the endocardial surface of the LA of two orthogonal apical views in a counterclockwise manner, and then, the LA endocardial border was automatically detected by the software (Fig. 1). We confirmed the border and adjusted manually if needed and started the 3DSTE analysis and time-global strain curves were generated. We also checked whether tracking was performed properly referring to the cine loops with tracking markers and shape of the strain waveform, and if it was insufficient, tried to re-analyze it, and excluded it when re-analyze was not successfully conducted. From the time-LA global strain curves, we measured LA peak global longitudinal strain during the reservoir phase (LA-LSR) and that during the atrial contraction phase (LA-LSCT), peak global circumferential strain during the reservoir phase (LA-CSR) and that during atrial contraction phase (LA-CSCT), and peak global area strain during the reservoir phase (LA-ASR) and that during the atrial contraction phase (LA-ASCT) [15] (Fig. 2). Similarly, from the time-LV global strain curves, we measured LV peak global longitudinal train (LV-LS), peak global circumferential strain (LV-CS), and peak global area strain (LV-AS). The absolute values of these strains were used in the present study. From the LA time-volume curve, maximal LA volume (LAVmax) and minimal LA volume (LAVmin) were measured to calculate the LA expansion index using the following equation:
From each time-LA strain curve, LA global longitudinal strain during the reservoir phase and that during atrial contraction phase (LA-LSR and LA-LSCT, respectively) (A); LA global circumferential strain during the reservoir phase and that during atrial contraction phase (LA-CSR and LA-CSCT, respectively) (B); and LA global area strain during the reservoir phase and that during atrial contraction phase (LA-ASR and LA-ASCT, respectively) (C) were measured
Statistical analysis
Statistical analysis was performed with standard statistical software (IBM SPSS Statistics ver. 25 for Windows, IBM SPSS, Chicago, IL, USA). Continuous variables are presented as mean ± standard deviation. Differences among the four groups were tested by one-way analysis of variance, and when a significant difference was detected, each difference between those two groups was tested using Tukey’s test. The relationship between a pair of parameters was assessed by linear correlation and regression analysis. Categorical variables were compared by the Fisher’s exact test at first, and differences between pairs of groups were tested using Ryan’s method. Receiver operating characteristic (ROC) analysis was used to evaluate the ability of LA global strain measurements for distinguishing HCM from HT-LVH. A multivariate linear regression analysis was performed to find the independent determinants of the LA strain parameters. The reproducibility of the LA global strain measurements was assessed in 15 randomly selected study subjects.
Results
Patient characteristics and conventional echocardiographic parameters
The clinical characteristics and conventional echocardiographic parameters of the HCM, HT-LVH, HT, and normal control groups are shown in Table 1. LV maximum wall thickness was significantly greater in the HCM group than in the other three groups, and was significantly greater in the HT-LVH group than in the control group. LA volume index was significantly greater in the HCM and HT-LVH groups than in the control group and did not significantly differ between the HCM and HT-LVH groups. E/e′ was significantly greater in the HCM group than in the control and HT groups, and was significantly greater in the HT-LVH group than in the control group. The percentage of subjects with LV diastolic dysfunction grade > 2 was higher in the HCM and HT-LVH groups than in the control and HT groups.
LA volumetric parameters derived from 3DSTE
Results for 3DSTE-derived LA volumetric parameters are shown in Table 2. LAVmax was significantly greater in the HCM and HT-LVH groups than in the control group. LAVmin was significantly greater in the HCM and HT-LVH groups than in the control group, and was significantly greater in the HCM group than in the HT group. LA expansion index was significantly lower in the HCM and HT-LVH groups than in the control group, and was significantly lower in the HCM group than in the HT group.
LA and LV strain parameters derived from 3DSTE
3DSTE-derived LA strain parameters are shown in Table 2 and Fig. 3. For the LA strains during the reservoir phase, LA-LSR was significantly lower in the HCM group than in the HT and control groups and did not significantly differ from that in the HT-LVH group. LA-LSR was significantly lower in the HT-LVH group than in the control group. LA-CSR was significantly lower in the HCM group than in the HT-LVH, HT, and control groups, and did not significantly differ among the HT-LVH, HT, and control groups. The results for LA-ASR were similar to those for LA-CSR. For the LA strains during the atrial contraction phase, LA-LSCT did not differ among groups. LA-CSCT was significantly lower in the HCM group than in the HT-LVH and HT groups, and did not significantly differ among the HT-LVH, HT, and control groups. The results for LA-ASCT were similar to those for LA-CSCT.
LV-LS was significantly lower in the HCM and HT-LVH groups than in the HT and control groups and did not significantly differ between the HCM and HT-LVH groups. LV-CS and LV-AS were not significantly different among the four groups.
The utilities of LA strain parameters for discriminating between patients with HT-LVH and those with HCM are summarized in Table 3. ROC analysis showed that LA-CSR, LA-CSCT, LA-ASR, and LA-ASCT had good diagnostic performance whereas LA-LSR and LA-LSCT did not. We also performed multivariate analyses to investigate whether decreased LA strains were independently related to HCM. Analyses with LA-LSR or LA-CSR as the objective variable and age, LA volume index, E/e′, LV-LS, and HCM as the explanatory variable showed that HCM was a significant independent predictor for LA-CSR but not for LA-LSR (supplemental Table).
Relationships between LV strains and LA strains during the reservoir phase
The correlations between LA strains (LA-LSR and LA-CSR) and LV strains (LV-LS and LV-CS) among all the study subjects are shown in Fig. 4. LA-LSR was significantly correlated with LV-LS but was not significantly correlated with LV-CS, while LA-CSR was significantly correlated with neither LV-LS nor LV-CS.
Reproducibility of measurements
Results of inter- and intra-observer reproducibility are summarized in Table 4. The interclass correlation coefficients for the inter- and intra-observer comparisons were excellent for both LA-LSR (0.95 and 0.93, respectively) and LA-CSR (0.90 and 0.94, respectively).
Discussion
LA myocardial expansion in the longitudinal and circumferential directions
In the present study, LA-LSR was significantly lower in the HCM and HT-LVH groups than in the control group, with no significant difference between the HCM and HT-LVH groups. On the other hand, LA-CSR and LA-ASR were significantly lower only in the HCM group compared to the other three groups. The usefulness of LA-CSR for distinguishing patients with HCM from those of HT-LVH was also investigated. The present study is the first to demonstrate such a unique feature of LA myocardial expansion abnormality in patients with HCM. Thus, the present results suggest that LA myocardial expansion during the reservoir phase was more seriously impaired in patients with HCM than in those with HT-LVH, and that the measurement of LA-CSR and LA-ASR using 3DSTE may be essential for an accurate and comprehensive understanding of decreased LA reservoir function in HCM patients.
Relationship between LA expansion and LV contraction
In our study subjects, LA-LSR was significantly correlated with LV-LS but not with LV-CS, while LA-CSR was correlated with neither LV-LS nor LV-CS. The LV and LA share the mitral annulus, which moves toward the apex during systole. In the longitudinal direction, the LA myocardial expansion after the onset of LV systole should be explained mainly by the passive extension owing to the pulling force of the powerful LV myocardial contraction. A few previous studies demonstrated the significant relationship between the LA expansion during the reservoir phase and LV contraction through an animal experiment using pressure–volume loop analysis [16] and an observational study using 2DSTE [5, 17]. When interpreting the LA-LSR, the LV-LS should be taken into account, however, this necessity is not widely recognized.
On the other hand, LA circumferential expansion should not be directly influenced by LV contraction; indeed, the LA-CSR was not correlated with any parameter of LV contraction in our results. This suggested that the impairment of LA circumferential expansion may more directly reflect the inherent abnormality in LA myocardial distensibility than that in longitudinal expansion. Thus, the LA-CSR and LA-ASR measured using 3DSTE may contribute to more profound insights into LA reservoir function in HCM patients.
Mechanism underlying decreased LA myocardial expansion during reservoir phase in HCM
It is generally thought that the hemodynamic burden to LA caused by LV systolic and diastolic dysfunction can cause LA functional abnormality, and several experimental studies have supported this [16,17,18,19]. However, the present study has shown a significant relationship between LA-LSR and LV-LS, suggesting that the longitudinal LA myocardial expansion may be distinctly influenced by LV longitudinal contraction. In our HT-LVH group, only LA-LSR was decreased together with LV-LS, and the impairment of longitudinal LA reservoir function in this group seems attributable to the impaired longitudinal LV contraction due to LVH rather than by the hemodynamic effect of LV dysfunction. In contrast, our HCM group had both LA-LSR and LA-CSR abnormalities in spite of there was no significant difference in the conventional LV systolic and diastolic parameters except for a relatively small difference in E/e′ between the HT-LVH and HCM groups. These results suggest that a more serious and intrinsic abnormality in the LA myocardium may be present in patients with HCM compared to those with HT-LVH. It is known that, in patients with HCM, myocardial pathological changes are seen not only in the ventricles but also in the atria [20]. In addition, a few studies have shown that the decreased LA strain is associated with histological alterations of the LA wall, such as myocardial fibrosis and endocardial thickening [21,22,23]. Thus, the deterioration of LA-CSR in our HCM group might be attributable to such pathological alterations of the LA myocardium rather than to the hemodynamic or mechanical burden to the LA due to LV dysfunction.
In the present study, LA-CSCT and LA-ASCT were also considered to be useful to assess deteriorated LA function in the HCM patients, however, they are thought to be more influenced by the preload for the LA (LA volume before atrial contraction) which is more influenced by LV relaxation, and afterload (late-diastolic LV stiffness). Therefore, we consider that the LA strains during the reservoir phase would be better to use for detecting deteriorated LA function.
Comparison with previous studies closely related to the present results
Domsik et al. reported that 3DSTE-derived LA longitudinal expansion was lower in their HCM patients than in the controls, whereas LA circumferential expansion and LA area strain were comparable [10]. One possible reason for the difference between their results and ours may stem from the difference in patient characteristics. Average age was lower in their study than in our HCM patients (49 ± 15 vs. 65 ± 14 years), and LA maximal volume was lower (66 ± 20 vs. 77 ± 22 mL). In addition, the LA-CSR mean and SD values for their HCM group were distinctly greater than those of ours (26.5 ± 16.5% vs. 20.0 ± 7.0%). These suggest that their study subjects may have included a wider range of HCM severity due to the inclusion of milder HCM cases.
Furukawa et al. reported that LA peak global area strain assessed by 3DSTE was comparable between hypertensive patients and controls [11]. However, they did not separate the hypertensive group by the presence or absence of LV hypertrophy, and they did not show the data for LA-LSR and LA-CSR. The present study demonstrated that LA myocardial expansion during the reservoir phase was generally preserved in hypertensive patients without LVH and was distinctly but only longitudinally reduced in hypertensive patients with LVH. These may also be among our new findings contributing to an understanding of LA reservoir function in hypertensive patients.
Clinical implications
The results of this study showed that the decrease in LA circumferential expansion, rather than in LA longitudinal expansion, may more distinctly reflect the decreased LA distensibility in HCM. Recently, several investigators have demonstrated that LA expansion is closely related to the prognosis of patients with cardiovascular diseases [24,25,26]. Mochizuki et al. reported that LA peak global circumferential strain and LA peak global area strain obtained by 3DSTE in patients with paroxysmal atrial fibrillation were predictors of atrial fibrillation recurrence after catheter ablation [27]. LA-CSR and LA-ASR obtained by 3DSTE may be useful to more accurately assess LA reservoir function and predict their prognosis in patients with LV hypertrophy as well as in a wider range of patients with heart diseases that can cause left heart failure. Further studies on 3DSTE analysis of LA expansion during the reservoir phase may lead to more profound insights into the mechanisms underlying ‘diastolic heart failure’ and into the prediction of prognosis in patients with heart failure with preserved ejection fraction.
Recently, several investigators have proposed the usefulness of adding the LA-LSR to the ASE/EACVI guidelines for detecting increased LV filling pressure [28, 29]. We expect that the LA-CSR may have equal or greater added value than the LA-LSR, but this remains to be further investigated.
Limitations
This study has several limitations. First, the number of cases was small. For this reason, in particular, the differences in strain parameters between asymmetric septal hypertrophy and apical hypertrophy remain unclear. Second, the present study was performed at a single center of a Japanese university hospital, and there might be some selection bias. Third, in the present study, six patients with HCM have had the LV outflow tract obstruction at rest (63 ± 29 mmHg). The possibility that LVOT obstruction has some effect on the LA strain parameters cannot be ruled out. Fourth, the accuracy of 3DSTE depends strongly on the quality of the obtained images. Although we initially recruited 97 patients, we then excluded 18 patients (19%) due to poor image quality. The low temporal resolution of 3DSTE may also be a problem. Yodwut et al. reported that LV strain measurements obtained using 3D images with a volume rate of 18 ± 2 vps were comparable to those obtained using 2DSTE with a frame rate of 62 ± 9 fps, and the temporal resolution of this study (16 to 40 vps) was considered acceptable [30]. 3D echocardiographic technology is advancing, and a further improvement in both 3D image quality and temporal resolution would overcome these problems for the clinical application of 3DSTE to LA.
Conclusion
Both longitudinal and circumferential LA expansions during the reservoir phase were reduced in patients with HCM, whereas only longitudinal LA expansion was reduced in those with HT-LVH. The deterioration of LA circumferential expansion may reflect a more serious and intrinsic decrease in LA myocardial distensibility in HCM patients. The measurement of LA circumferential and area strains using 3DSTE would be useful to obtain a more accurate understanding of LA reservoir function in patients with LV hypertrophy, including those with HCM.
Data availability
The data is available, and permission might be sought per reasonable request.
References
Sanada H, Shimizu M, Sugihara N, Shimizu K, Ino H, Takeda R (1993) Increased left atrial chamber stiffness in hypertrophic cardiomyopathy. Br Heart J 69:31–35. https://doi.org/10.1136/hrt.69.1.31
Anwar AM, Soliman OI, Nemes A, Geleijnse ML, ten Cate FJ (2008) An integrated approach to determine left atrial volume, mass and function in hypertrophic cardiomyopathy by two-dimensional echocardiography. Int J Cardiovasc Imaging 24:45–52. https://doi.org/10.1007/s10554-007-9224-x
Järvinen VM, Kupari MM, Poutanen VP, Hekali PE (1996) A simplified method for the determination of left atrial size and function using cine magnetic resonance imaging. Magn Reson Imaging 14:215–226. https://doi.org/10.1016/0730-725x(95)02098-e
Paraskevaidis IA, Panou F, Papadopoulos C, Farmakis D, Parissis J, Ikonomidis I, Rigopoulos A, Iliodromitis EK, Th Kremastinos D (2009) Evaluation of left atrial longitudinal function in patients with hypertrophic cardiomyopathy: a tissue Doppler imaging and two-dimensional strain study. Heart 95:483–489. https://doi.org/10.1136/hrt.2008.146548
Roşca M, Popescu BA, Beladan CC, Călin A, Muraru D, Popa EC, Lancellotti P, Enache R, Coman IM, Jurcuţ R, Ghionea M, Ginghină C (2010) Left atrial dysfunction as a correlate of heart failure symptoms in hypertrophic cardiomyopathy. J Am Soc Echocardiogr 23:1090–1098. https://doi.org/10.1016/j.echo.2010.07.016
Badran HM, Faheem N, Elnoamany MF, Kenawy A, Yacoub M (2015) Characterization of left atrial mechanics in hypertrophic cardiomyopathy and essential hypertension using vector velocity imaging. Echocardiography 32:1527–1538. https://doi.org/10.1111/echo.12885
Kowallick JT, Kutty S, Edelmann F, Chiribiri A, Villa A, Steinmetz M, Sohns JM, Staab W, Bettencourt N, Unterberg-Buchwald C, Hasenfuß G, Lotz J, Schuster A (2014) Quantification of left atrial strain and strain rate using Cardiovascular Magnetic Resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson 16:60. https://doi.org/10.1186/s12968-014-0060-6
Mondillo S, Cameli M, Caputo ML, Lisi M, Palmerini E, Padeletti M, Ballo P (2011) Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr 24:898–908. https://doi.org/10.1016/j.echo.2011.04.014
Açar G, Bulut M, Arslan K, Alizade E, Ozkan B, Alici G, Tanboga IH, Yazicioğlu MV, Akcakoyun M, Esen AM (2013) Comparison of left atrial mechanical function in nondipper versus dipper hypertensive patients: a speckle tracking study. Echocardiography 30:164–170. https://doi.org/10.1111/echo.12023
Domsik P, Kalapos A, Chadaide S, Sepp R, Hausinger P, Forster T, Nemes A (2014) Three-dimensional speckle tracking echocardiography allows detailed evaluation of left atrial function in hypertrophic cardiomyopathy: insights from the MAGYAR-Path Study. Echocardiography 31:1245–1252. https://doi.org/10.1111/echo.12568
Furukawa A, Ishii K, Hyodo E, Shibamoto M, Komasa A, Nagai T, Tada E, Seino Y, Yoshikawa J (2016) Three-dimensional speckle tracking imaging for assessing left atrial function in hypertensive patients with paroxysmal atrial fibrillation. Int Heart J 57:705–711. https://doi.org/10.1536/ihj.16-121
Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H (2014) 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 35:2733–2779. https://doi.org/10.1093/eurheartj/ehu284
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28:1-39.e14. https://doi.org/10.1016/j.echo.2014.10.003
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29:277–314. https://doi.org/10.1016/j.echo.2016.01.011
Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, D’Hooge J, Donal E, Fraser AG, Marwick T, Mertens L, Popescu BA, Sengupta PP, Lancellotti P, Thomas JD, Voigt JU (2018) Industry representatives; Reviewers: this document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 19:591–600. https://doi.org/10.1093/ehjci/jey042
Barbier P, Solomon SB, Schiller NB, Glantz SA (1999) Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation 100:427–436. https://doi.org/10.1161/01.cir.100.4.427
Inoue K, Khan FH, Remme EW, Ohte N, García-Izquierdo E, Chetrit M, Moñivas-Palomero V, Mingo-Santos S, Andersen ØS, Gude E, Andreassen AK, Wang TKM, Kikuchi S, Stugaard M, Ha JW, Klein AL, Nagueh SF, Smiseth OA (2021) Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur Heart J Cardiovasc Imaging 23:61–70. https://doi.org/10.1093/ehjci/jeaa415
Blume GG, Mcleod CJ, Barnes ME, Seward JB, Pellikka PA, Bastiansen PM, Tsang TS (2011) Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr 12:421–430. https://doi.org/10.1093/ejechocard/jeq175
Bisbal F, Baranchuk A, Braunwald E, Bayés de Luna A, Bayés-Genís A (2020) Atrial failure as a clinical entity: JACC review topic of the week. J Am Coll Cardiol 75:222–232. https://doi.org/10.1016/j.jacc.2019.11.013
Ohtani K, Yutani C, Nagata S, Koretsune Y, Hori M, Kamada T (1995) High prevalence of atrial fibrosis in patients with dilated cardiomyopathy. J Am Coll Cardiol 25:1162–1169. https://doi.org/10.1016/0735-1097(94)00529-y
Cameli M, Lisi M, Righini FM, Massoni A, Natali BM, Focardi M, Tacchini D, Geyer A, Curci V, Di Tommaso C, Lisi G, Maccherini M, Chiavarelli M, Massetti M, Tanganelli P, Mondillo S (2013) Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol 111:595–601. https://doi.org/10.1016/j.amjcard.2012.10.049
Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, Rao SN, Blauer J, Fish EN, Dibella EV, Macleod RS, McGann C, Litwin SE, Marrouche NF (2010) Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging 3:231–239. https://doi.org/10.1161/CIRCIMAGING.109.865683
Yuda S (2021) Current clinical applications of speckle tracking echocardiography for assessment of left atrial function. J Echocardiogr 19:129–140. https://doi.org/10.1007/s12574-021-00519-8
Kurt M, Wang J, Torre-Amione G, Nagueh SF (2009) Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging 2:10–15. https://doi.org/10.1161/CIRCIMAGING.108.813071
Cameli M, Lisi M, Mondillo S, Padeletti M, Ballo P, Tsioulpas C, Bernazzali S, Maccherini M (2010) Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound 8:14. https://doi.org/10.1186/1476-7120-8-14
Galli E, Fournet M, Chabanne C, Lelong B, Leguerrier A, Flecher E, Mabo P, Donal E (2016) Prognostic value of left atrial reservoir function in patients with severe aortic stenosis: a 2D speckle-tracking echocardiographic study. Eur Heart J Cardiovasc Imaging 17:533–541. https://doi.org/10.1093/ehjci/jev230
Mochizuki A, Yuda S, Fujito T, Kawamukai M, Muranaka A, Nagahara D, Shimoshige S, Hashimoto A, Miura T (2017) Left atrial strain assessed by three-dimensional speckle tracking echocardiography predicts atrial fibrillation recurrence after catheter ablation in patients with paroxysmal atrial fibrillation. J Echocardiogr 15:79–87. https://doi.org/10.1007/s12574-017-0329-5
Smiseth OA, Morris DA, Cardim N, Cikes M, Delgado V, Donal E, Flachskampf FA, Galderisi M, Gerber BL, Gimelli A, Klein AL, Knuuti J, Lancellotti P, Mascherbauer J, Milicic D, Seferovic P, Solomon S, Edvardsen T, Popescu BA (2022) Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 23:e34–e61. https://doi.org/10.1093/ehjci/jeab154
Venkateshvaran A, Tureli HO, Faxén UL, Lund LH, Tossavainen E, Lindqvist P (2022) Left atrial reservoir strain improves diagnostic accuracy of the 2016 ASE/EACVI diastolic algorithm in patients with preserved left ventricular ejection fraction: insights from the KARUM haemodynamic database. Eur Heart J Cardiovasc Imaging. https://doi.org/10.1093/ehjci/jeac036
Yodwut C, Weinert L, Klas B, Lang RM, Mor-Avi V (2012) Effects of frame rate on three-dimensional speckle-tracking-based measurements of myocardial deformation. J Am Soc Echocardiogr 25:978–985. https://doi.org/10.1016/j.echo.2012.06.001
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by YY, KO and MA. The first draft of the manuscript was written by YY and KO, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved as a retrospective observational study by the Research Ethics Committee of Hokkaido University Hospital (No. 019-0118). Instead of obtaining informed consent, the objectives and methods of the present study were shared with the public both through our institution’s website and on a physical bulletin board; patients who did not wish to participate could request their data be deleted from the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yanagi, Y., Okada, K., Kaga, S. et al. Difference in left atrial myocardial dynamics during reservoir phase between hypertrophic cardiomyopathy and hypertensive heart determined using three-dimensional speckle tracking echocardiography. Int J Cardiovasc Imaging 38, 1781–1791 (2022). https://doi.org/10.1007/s10554-022-02604-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-022-02604-0