Abstract
In patients with symptomatic severe aortic stenosis (AS), those who experienced readmission due to heart failure after transcatheter aortic valve replacement (TAVR) showed poor prognosis. Furthermore, poor B-type natriuretic peptide (BNP) improvement is associated with increased morbidity and mortality. However, little is known about the clinical parameters related to the change in BNP levels after TAVR procedure. This study population consisted of 127 consecutive patients of symptomatic severe AS with preserved ejection fraction (EF) who underwent transfemoral TAVR (TF-TAVR). Comprehensive transthoracic echocardiography was performed prior to the day of TF-TAVR. BNP was measured serially before and 1 year after TF-TAVR. The median BNP level was significantly decreased from 252.5 pg/ml to 146.8 pg/ml in all 127 patients 1 year after TF-TAVR (P < 0.01). However, the patients could be divided into 2 groups according to decrease (72%) or increase (28%) in plasma BNP level. Multivariate logistic regression analysis revealed that Aortic valve (AV) peak velocity, pre-procedural BNP, and larger left atrial volume index (LAVI) were found to be an independent predictor of increased BNP level 1 year after TAVR (OR 0.55, 95% CI 0.38–0.77; P < 0.01). LAVI were negatively correlated with the change in BNP level before and 1 year after TAVR (r = 0.47, P < 0.01). The ROC analysis demonstrated that 52.9 ml/m2 was the optimal cut-off value of LAVI for decreasing BNP 1 year after TAVR (area under the curve 0.69) with 64% sensitivity and 70% specificity. In addition to AV peak velocity and pre-procedural BNP, LAVI independently predicts future improvement of BNP levels 1 year after TAVR. Our findings indicate an additive predictive value of assessment of LAVI before TAVR procedure for risk stratification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transcatheter aortic valve replacement (TAVR) is becoming an alternative intervention to surgical aortic valve replacement (SAVR) for high-risk, and even low-risk, patients with severe symptomatic aortic stenosis (AS) [1,2,3]. Although TAVR has comparable outcomes to SAVR, patients requiring readmission after TAVR have a poor prognosis [4, 5]. Congestive heart failure is the most common cardiac cause of readmission [4]. Therefore, it is clinically important to identify patients at high risk for heart failure after TAVR.
B-type natriuretic peptide (BNP) is a non-invasive biomarker for the diagnosis and management of patients with heart failure or various other cardiac diseases [6, 7]. In patients who undergo TAVR to treat AS, elevated BNP levels at baseline or discharge are associated with poor outcomes [8, 9]. Changes in BNP levels before and after TAVR, rather than a single BNP measurement, can more accurately predict prognosis [10,11,12]. Patients with poor improvement in BNP levels after TAVR have less symptomatic improvement and higher long-term mortality rates than patients with lower BNP levels [10,11,12]. Ideally, plasma BNP levels should decrease within the first year after TAVR and remain stable thereafter [2, 13]. However, around 35–40% of patients show persistent elevated plasma BNP levels at their 1-year follow-up after TAVR, and these patients have higher rates of morbidity and mortality [10, 11]. Therefore, it is important to remeasure BNP levels at 1-year follow-up appointments to predict prognosis in AS patients who have undergone TAVR. In addition, persistent elevated plasma BNP levels after TAVR suggests that the increased BNP level before TAVR in patients with severe AS is affected not only by pressure overload but also by other factors. However, little is currently known about which clinical parameters predict changes in BNP levels in patients with severe AS after TAVR. We hypothesized that transthoracic echocardiography (TTE) might be useful to characterize the differences in cardiac imaging between the patients with decrease or increase BNP level after TAVR procedure. In the present study, we assess the clinical factors associated with serial changes in BNP levels before and after transfemoral TAVR (TF-TAVR) and at the 1-year follow-up timepoint.
Methods
Study population
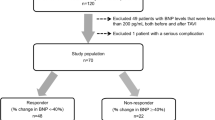
This study was a single-center prospective observational study undertaken at Osaka City University Hospital. We examined 237 consecutive symptomatic severe AS patients who underwent TAVR between January 2016 and January 2019. To minimize the influence on serial BNP levels, we excluded 34 patients who underwent TAVR by non-TF approaches, 33 patients with an EF less than 50%, 25 patients with more than moderate mitral regurgitation (MR), and 18 patients who were not able to carry out clinical follow-up for more than 1 year after TAVR. In this study, there were no case of severe aortic regurgitation (AR), 14 cases of moderate AR and 5 cases of bicuspid aortic valve. All cases were included in this study. Paradoxical low-flow low-gradient AS characterized by EF ≥ 50% at low-flow low-gradient (i.e. Stroke Volume Index (SVI) < 35 ml/m2, Mean Gradient (MG) < 40 mmHg and Peak Velocity (PV) < 4 m/s) were included (n = 8), and low-flow low-gradient AS (EF < 50% and MG < 40 mmHg, PV < 4 m/s, SVI < 35 ml/m2) were excluded (n = 33). With these exclusions, we enrolled 127 patients with preserved EF who underwent TF-TAVR (Fig. 1).
Transthoracic echocardiography (TTE) was performed the day before TAVR, and laboratory data was measured both before and 1 year after TAVR. All data shown in the tables and figures were collected prospectively from patient records. Clinical data including patient characteristics and echocardiographic data were prospectively recorded.
Written informed consent was obtained from each patient included in this study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and the study protocol was approved by the hospital ethics committee of the Osaka City University Graduate School of Medicine (Approval Number: 2021-064).
Transthoracic echocardiography
Comprehensive two-dimensional TTE was performed within the seven days preceding the TAVR procedure using an iE33 (Philips Medical Systems, Andover, MA, USA), Vivid E9 (GE Healthcare, Milwaukee, WI, USA), or Aplio 500 (Canon Medical Systems Corporation, Tochigi, Japan) machine equipped with a high-frequency transducer. Left ventricle end-diastolic diameter (LVDD), left ventricular end-systolic diameter (LVSD), interventricular septal thickness (IVS), and posterior wall thickness (PW) were measured in accordance with published guidelines [14]. Left ventricular mass (LVM) was calculated using the formula (LVM = 0.80 × 1.04 × [ (LVDD + IVS + PW)3 − LVDD3] + 0.6 g). Also, left atrial volume (LAV) was measured using the biplane method of disks at end-systole just before mitral valve opening. In each view, the LA wall was traced, excluding the LA appendage and pulmonary veins. The intra-observer variability of LAV was determined by measuring the experienced echocardiographer (A.I) twice on different days using the same images. The inter-observer variability of LAV was determined by using the same image to measure the LAV of two experienced echocardiographers (A.I., M.O.) who were blinded and measured LAV using the same images. LVM and LA volume measurements were indexed to body surface area to calculate the left atrial volume index (LAVI). We evaluated left ventricular EF with the biplane-modified Simpson method [15].
Using the pulse wave Doppler method, the peak velocity of diastolic early trans-mitral Doppler flow (E) was measured by placing a sample volume at the opening levels of the mitral valve leaflet tips. Tissue Doppler velocity during early diastole (e′) was measured from the septal corner of the mitral annulus in the apical four-chamber view. Doppler measurements were obtained by averaging data from three consecutive cardiac cycles in the case of sinus rhythm and five consecutive cardiac cycles in the case of atrial fibrillation (AF), and E/e′ ratios were calculated.
Multiple transducer positions were used to record aortic jet velocities to avoid angle-related errors and the aortic valve area was calculated using the continuity equation method [15]. Severe AS was defined as a peak aortic valve jet velocity of > 4.0 m/s, a mean aortic valve gradient (AV mean PG) > 40 mmHg, or an aortic valve area (AVA) < 1.0 cm [2, 14]. Severity of MR was graded as none, trivial, mild, moderate, or severe primarily by MR jet area, and, if necessary, other echocardiographic methods, including vena contracta, proximal isovelocity surface area, peak E velocity of the trans-mitral inflow, or pulmonary venous flow velocity data (S/D ratio), were incorporated [14].
Plasma BNP measurements
Peripheral venous blood samples for laboratory analysis were drawn before and 1 year after TAVR. Plasma BNP concentration was measured by chemiluminescent immunoassay (BNP-JP Abbott instrument; Abbott Japan, Tokyo, Japan).
Statistical analysis
Continuous variables were summarized using medians and interquartile range (quartiles 1–3), and categorical variables were summarized using counts and percentages. When two groups were compared, the unpaired t-test or Mann–Whitney U test was used, as appropriate. Categorical variables were compared using the χ2 test or Fisher’s exact test. Association of clinical factors with decreased BNP 1 year after TAVR was evaluated by multiple logistic regression analysis after adjustment for significant variables with P < 0.01 on univariate analysis. Spearman’s rank correlation coefficient was used to analyze the correlation between LAVI and the change in BNP levels, before and 1 year after TAVR. The validity of the change in BNP levels before and 1 year after TF-TAVR for estimating associated clinical factors was evaluated using receiver operating characteristics (ROC) curves. We used the Kappa statistic to calculate inter and intra variabilities of LAV. P-values < 0.05 were considered statistically significant. All statistical analyses were performed with EZR [16], which is a modified version of R commander designed to add statistical functions frequently used in biostatistics.
Results
Clinical baseline patient characteristics are listed in Table 1. In the total study population, the median patient age was 84 (quartile 81–87) years, and 38/127 (30%) patients were male. The median pre-TAVR BNP level was 252.5 pg/ml (quartile 74.3–352.1) and the median estimated glomerular filtration rate (eGFR) was 51.7 ml/min/1.73 m2 (quartile 38.6–63.2). There were 9 patients (7%) received permanent pacemaker implantation after TAVR, 7 patients (8%) in BNP decrease group and 2 patients (6%) in BNP increase group. There was no difference between two groups.
The median BNP level in the total population of 127 patients significantly decreased from 252.5 to 146.8 pg/ml 1 year after TF-TAVR (P < 0.01) (Fig. 2). However, the patients could be divided into two groups according to whether their plasma BNP levels decreased (91/127, 72%) 9 or increased (36/127, 28%) 1 year after TF-TAVR. Representative TTE images before TAVR in clinical cases with decreased or increased plasma BNP levels were shown in Fig. 3. The characteristics of patients with decreased or increased BNP levels 1 year after TF-TAVR were shown in Table 1. There were no significant differences in the frequency of hypertension, eGFR and AF between the two groups. Patients in the decreased BNP group had higher pre-TAVR BNP levels (294.2 (101.5–438.1) vs. 147.2 (46.5–222.2); P < 0.01) and higher STS scores (9.4 (5.7–11.8) vs. 6.6 (4.8–8.9); P = 0.03) than those with increased BNP levels.
Representative TTE images before TAVR in clinical cases with decreased or increased plasma BNP levels. Clinical cases with increased plasma BNP levels. In this case, LAVI was 88 ml/m2 and BNP was 183.2 pg/dl before TAVR, and the BNP level was 342.2 pg/dl 1 year after TAVR. A Clinical cases with decreased plasma BNP levels. In this case, LAVI was 44 ml/m2 and BNP was 114.2 pg/dl before TAVR, and the BNP level was 25.1 pg/dl 1 year after TAVR. B Clinical cases with decreased plasma BNP levels. In this case, LAVI was 44 ml/m2 and BNP was 114.2 pg/dl before TAVR, and the BNP level was 25.1 pg/dl one year after TAVR. BNP B-type natriuretic peptide; LAVI left atrial volume index; TAVR transcatheter aortic valve replacement; TTE transthoracic echocardiography
Echocardiographic data for all patients are shown in Table 2. The intra-observer and the inter-observer agreement were excellent for LAV (κ = 0.92, κ = 0.82, respectively). There were no significant differences in LV end-diastolic and end-systolic dimensions, LV wall thickness, E-wave velocities on trans-mitral doppler flow, e′ velocities on TDI and the E/e′ ratio between the two groups. There were 10 cases of moderate AR and 5 cases of bicuspid aortic valve in BNP decrease group, and 2 patients of moderate AR and 1 case of bicuspid aortic valve in BNP increase group. There was no significant difference between two groups. However, patients with decreased BNP levels 1 year after TF-TAVR had smaller LAVIs (48.7 (40.5–56.5) vs. 53.1 (46.3–60.9); P = 0.02), smaller Aortic Valve areas (AVA) (0.65 (0.56–0.72) vs. 0.71 (0.64–0.75); P = 0.02), greater Aortic Valve (AV) mean pressure gradients (49.5 (39.0–68.0) vs. 42.1 (36.5–52.3); P = 0.02), and higher AV peak velocities (4.7 (4.2–5.4) vs. 4.35 (4.0–4.8); P = 0.01) than patients with increased BNP levels.
To identify the factors that predict improvement in BNP levels after TAVR, we performed logistic regression analysis. In the univariate logistic regression analysis, higher pre-TAVR BNP (OR 4.98, 95% CI 1.99–12.6; P < 0.01), smaller LAVI (per 10 ml/m2) (OR 0.67, 95% CI 0.50–0.89; P < 0.01) and higher AV peak velocity (OR 2.19, 95% CI 1.22–3.93; P < 0.01) were significant predictors of decreased BNP levels 1 year after TAVR (P < 0.01) (Table 3). Multivariate logistic regression analysis after adjustment for pre-TAVR BNP and AV peak velocity revealed higher LAVI to be an independent predictor of increased BNP levels 1 year after TAVR (OR 0.55, 95% CI 0.38–0.77; p < 0.01) (Table 3).
We assessed the correlation between LAVI and the change in BNP levels before and 1 year after TAVR. LAVI negatively correlated with the change in BNP levels before and 1 year after TAVR (r = 0.47, P < 0.01) (Fig. 4A). ROC analysis demonstrated that 52.9 ml/m2 was the optimal LAVI cut-off value for decreased BNP 1 year after TAVR (area under the curve 0.69) with 64% sensitivity and 70% specificity (Fig. 4B).
A Correlation between LAVI and the change in BNP levels before and 1 year after TAVR (r = 0.47, p < 0.01). B ROC curve of LAVI as a predictor of decreased BNP levels 1 year after TF-TAVR. The optimal LAVI cut-off value was 52.9 ml/m2 for predicting decreased BNP 1 year after TF-TAVR (area under the curve 0.69). BNP B-type natriuretic peptide; LAVI left atrial volume index; TAVR transcatheter aortic valve replacement
Discussion
The key findings of our study in 127 consecutive patients with symptomatic severe AS and preserved EF who underwent TF-TAVR were: (1) in the total patient population, the median BNP level significantly decreased 1 year after the procedure, however, the patients could be divided into two groups according to whether their plasma BNP levels decreased (72%) or increased (28%); (2) pre-TAVR BNP levels, AV peak velocity and higher LAVI were independent predictors of increased BNP levels 1 year after TAVR; (3) LAVI negatively correlated with the change in BNP levels before and 1 year after TAVR; and (4) 52.9 ml/m2 was the optimal LAVI cut-off value for decreased BNP 1 year after TAVR. It may be suggested that this is a new simple echocardiographic parameter that is clinically useful.
It has been reported that elevated BNP levels correlate with AS severity [17]. Intervention to treat AS, regardless of whether it is SAVR or TAVR, can significantly improve BNP levels [17, 18]. In this study, the median BNP levels of the total population of 127 patients were significantly improved 1 year after TF-TAVR. Patients with decreased BNP levels at 1 year after TAVR had significantly higher AS severity indices and higher pre-TAVR BNP levels than patients with increased BNP levels 1 year after TAVR. The decrease in BNP levels may reflect hemodynamic improvement after TAVR because successful TF-TAVR reduces afterload, which causes LV hypertrophy [19], and LV filling pressures, which contribute to increased BNP levels [20].
However, in the patients with increased BNP levels, there may be other factors involved besides the severity of their AS. A variety of factors including heart failure, acute coronary syndromes, cardiac hypertrophy and various other clinical factors can affect BNP levels in patients with severe AS [18, 20]. Previous studies regarding to BNP level and left-sided cambers remodeling reported that increase BNP level after TAVR was related to the effects of residual more than mitral regurgitation, atrial fibrillation, and neurohumoral factors [10, 11]. Regardless of those factors, our data suggest that high LAVI is associated with elevated BNP levels. This imaging parameter (LAVI > 52.9 ml/m2) may be not only new, but also simple and potentially clinically useful. Left atrial enlargement is common in patients with AS [21] and patients with severe AS have greater LAVI compared with patients with mild AS [22, 23]. Increased LA volume in AS patients represents a chronic increase in left ventricular diastolic pressure caused by both valvular damage and the LV remodeling process [24]. In the present study, larger LAVI was an independent predictor of poor improvement in BNP levels. A previous study has shown that LAVI represents left atrial function or LV diastolic function [21]. Typically, LA function has 3 phases, serving as a reservoir in systole, as a conduit in early diastole, and as a booster pump in late diastole [25]. There is a close interaction between LA and LV function in each phase of LA function [26]. The longer the left ventricular end-diastolic pressure is high, the more the left atrial enlargement progresses, so the left atrial size is a cumulative measure of the degree and duration of left ventricular diastolic dysfunction [26]. If the left atrium in patients with severe AS becomes too large due to sustained overload, atrial function or LV diastolic function may not improve even after appropriate treatment. Our present findings suggest that, even after successful TF-TAVR, patients who have a high LAVI may not have improved left atrial function or LV diastolic function, and this could be associated with poor improvement in BNP levels.
This concept is supported by a recent report showing that large LAVI in patients with severe AS is a strong predictor of death under medical and surgical treatment despite the proven beneficial effects of these treatments [27]. Furthermore, consistent with our findings, the report also showed that severe LA enlargement (LAVI ≥ 50 ml/m2) is associated with a substantial increase in the risk of all-cause mortality during follow-up [27]. These data suggest that patients with larger LAVI might be considered as high-risk even after successful TF-TAVR. This study also may suggest that excessive LA enlargement (LAVI ≥ 52.9 ml/m2) is a clinically useful marker of future BNP increase in severe AS patients with preserved EF even after successful TF-TAVR. Therefore, systematic evaluation of LAVI in severe AS patients with preserved EF may be a useful imaging parameter to help make future clinical decisions. LAVI may have an additional adjunctive role when combined with BNP in risk stratification of patients with severe AS before a TF-TAVR procedure.
Conclusion
In addition to AV peak velocity and pre-procedural BNP levels, LAVI independently predicts future improvement in BNP levels 1 year after TAVR. This finding indicates an additive predictive value of LAVI assessment before a TF-TAVR procedure and a complementary role of BNP and LAVI for risk stratification in these patients. Careful monitoring of these high-risk patients may avoid hospitalization for heart failure and consequently reduce mortality.
Study limitations
This study has several limitations. First, this is a single center study and includes a Japanese-only patient population. Therefore, further prospective studies are necessary to assess whether there is a similar association between the change in BNP levels and LAVI in patients with preserved EF who undergo TF-TAVR and who are of different ethnic backgrounds. Second, although we adjusted for variables that may affect BNP levels, it is still possible that some confounding factors may have affected our results. Third, although this study population consisted of a series of consecutive patients who were referred for clinically indicated TAVR, a potential selection bias may confound our results. Forth, in this study we focused on predicting the postoperative clinical course with the preoperative Imaging parameter and could not show the data of LAVI after 1 year of TAVR. Fifth, regarding the timing of echo evaluation after TAVR, some studies have been done early [11], while others have been done after one year, it may be still controversial [13, 28]. Fifth, although two-dimensional (2D) echocardiography is currently standard method for LAV measurement [29], 2D echocardiographic measurement of LAV may not be accurate in some cases [30]. Previous studies have shown that LAV assessed by three-dimensional (3D) echocardiography is a better correlate of LV diastolic function [31]. 3D echocardiography would be ideal for the measurement of LAV. Prospective studies using 3D echocardiography will be necessary in the future. Finally, we were not able to show the data of the control subjects in this study. In the future, it will be necessary to verify the results with a control group.
References
Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson JL, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ, PARTNER Trial Investigators (2011) Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 364:2187–2198
Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock AJ, Alu MC, Webb JG, Smith CR, PARTNER 3 Investigators (2019) Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 380:1695–1705
Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, Webb JG, Yoon SH, Trento A, Svensson LG, Herrmann HC, Szeto WY, Miller DC, Satler L, Cohen DJ, Dewey TM, Babaliaros V, Williams MR, Kereiakes DJ, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Brown DL, Fearon WF, Russo MJ, Pibarot P, Hahn RT, Jaber WA, Rogers E, Xu K, Wheeler J, Alu MC, Smith RC, Leon MB, PARTNER 2 Investigators (2020) Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med 382:799–809
Durand E, Doutriaux M, Bettinger N, Tron C, Fauvel C, Bauer F, Dacher JN, Bouhzam N, Litzler PY, Cribier A, Eltchaninoff H (2017) Incidence, prognostic impact, and predictive factors of readmission for heart failure after transcatheter aortic valve replacement. JACC Cardiovasc Interv 10:2426–2436
Jalava MP, Laakso T, Virtaen M, Niemela M, Ahvenvaara T, Tauriainen T, Maaranen P, Husso A, Kinnunen EM, Dahlbacka S, Jaakkola J, Airaksinen J, Anttila V, Rosato S, D’Errigo P, Savontaus M, Laine M, Makikallio T, Valtola A, Raivio P, Eskola M, Biancari F (2020) Transcatheter and surgical aortic valve replacement in patients with recent acute heart failure. Ann Thorac Surg 109:110–117
Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, Pacher R (2002) B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation 105:2392–2397
Volpe M, Rubattu S, Burnett J Jr (2014) Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J 35:419–425
Chen S, Redfors B, O’Neill BP, Clavel MA, Pibarot P, Elmariah S, Nazif T, Crowley A, Ben-Yehuda O, Finn MT, Alu MC, Vahl TP, Kodali S, Leon MB, Lindman BR (2019) Low and elevated B-type natriuretic peptide levels are associated with increased mortality in patients with preserved ejection fraction undergoing transcatheter aortic valve replacement: an analysis of the PARTNER II trial and registry. Eur Heart J 41:958–969
Mizutani K, Hara M, Iwata S, Murakami T, Shibata T, Yoshiyama M, Naganuma T, Yamanaka F, Higashimori A, Tada N, Takagi K, Araki M, Ueno H, Tabata M, Shirai S, Watanabe Y, Yamamoto M, Hayashida K (2017) Elevation of B-type natriuretic peptide at discharge is associated with 2-year mortality after transcatheter aortic valve replacement in patients with severe aortic stenosis: insights from a multicenter prospective OCEAN-TAVI (optimized transcatheter valvular intervention-transcatheter aortic valve implantation) registry. J Am Heart Assoc 6:e006112
Ribeiro HB, Urena M, Le Ven F, Nombela-Franco L, Allende R, Clavel MA, Dahou A, Côté M, Laflamme J, Laflamme L, DeLarochellière H, DeLarochellière R, Doyle D, Dumont E, Bergeron S, Pibarot P, Rodés-Cabau J (2014) Long-term prognostic value and serial changes of plasma N-terminal prohormone B-type natriuretic peptide in patients undergoing transcatheter aortic valve implantation. Am J Cardiol 113:851–859
O’Neill BP, Guerrero M, Thourani VH, Kodali S, Heldman A, Williams M, Xu K, Pichard A, Mack M, Babaliaros V, Herrmann HC, Webb J, Douglas PS, Leon MB, O’Neill WW (2015) Prognostic value of serial B-type natriuretic peptide measurement in transcatheter aortic valve replacement (from the PARTNER Trial). Am J Cardiol 115:1265–1272
Koskinas KC, O’Sullivan CJ, Heg D, Praz F, Stortecky S, Pilgrim T, Buellesfeld L, Jüni P, Windecker S, Wenaweser P (2015) Effect of B-type natriuretic peptides on long-term outcomes after transcatheter aortic valve implantation. Am J Cardiol 116:1560–1565
Sato K, Kumar A, Krishnaswamy A, Mick S, Desai MY, Griffin BP, Kapadia SR, Popović ZB (2018) B-type natriuretic peptide is associated with remodeling and exercise capacity after transcatheter aortic valve replacement for aortic stenosis. Clin Cardiol 42:270–276
Baumagarter H, Falk V, Bax JJ, Bonis MD, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Munoz DR, Rosenhek R, Sjogren J, Mas PT, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL (2017) 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 38:2739–2391
Mitchell C, Rahko P, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA, Velazquez EJ (2019) Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 32:1–64
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl 48:452–458
Gerber IL, Stewart RA, Legget ME, West TM, French RL, Sutton TM, Yandle TG, French JK, Richards AM, White HD (2003) Increased plasma natriuretic peptide levels reflect symptom onset in aortic stenosis. Circulation 107:1884–1890
Clavel MA, Malouf J, Michelena HI, Suri RM, Jaffe AS, Mahoney DW, Enriquez-Sarano M (2014) B-type natriuretic peptide clinical activation in aortic stenosis: impact on long-term survival. J Am Coll Cardiol 63(19):2016–2025
Lorell BH, Carabello BA (2000) Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 102:470–479
Daniels LB, Maisel AS (2007) Natriuretic peptides. J Am Coll Cardiol 50:2357–2368
Dalsgaard M, Egstrup K, Wachtell K et al (2008) Left atrial volume in patients with asymptomatic aortic valve stenosis (the Simvastatin and Ezetimibe in Aortic Stenosis study). Am J Cardiol 101:1030–1034
Dalsgaard M, Egstrup K, Wachtell K, Gerdts E, Cramariuc D, Kjaergaard J, Hassager C (2003) Left atrial volume in patients with asymptomatic aortic valve stenosis (the Simvastatin and Ezetimibe in Aortic Stenosis study). J Am Coll Cardiol 42:1206–1207
Mosquera VX, Bouzas-Mosquera A, González-Barbeito M, Bautista-Hernandez V, Muñiz J, Alvarez-García N, Cuenca-Castillo JJ (2017) Indexed left atrial size predicts all-cause and cardiovascular mortality in patients undergoing aortic valve surgery. J Thorac Cardiovasc Surg 153:1275–1284
Morimoto J, Hozumi T, Takemoto K, Wada T, Maniwa N, Kashiwagi M, Shimamura K, Shiono Y, Kuroi A, Yamano T, Yamaguchi T, Matsuo Y, Kitabata H, Ino Y, Kubo T, Tanaka A, Nishimura Y, Akasaka T (2019) preoperative left atrial minimum volume as a surrogate marker of postoperative symptoms in senile patients with aortic stenosis who underwent surgical aortic valve replacement. J Cardiol 74:366–371
Barbier P, Solomon SB, Schiller NB, Glantz SA (1999) Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation 100:427–436
Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP (2019) Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state-of-the-art review. J Am Coll Cardiol 73(15):1961–1977
Rusinaru D, Bohbot Y, Kowalski C, Ringle A, Maréchaux S, Tribouilloy C (2017) Left atrial volume and mortality in patients with aortic stenosis. J Am Heart Assoc 6:e006615
Ben-Dor I, Minha S, Barbash IM, Aly O, Dvir D, Deksissa T, Okubagzi O, Torguson R, Lindsay J, Satler LF, Pichard AD, Waksman R (2013) Correlation of brain natriuretic peptide levels in patients with severe aortic stenosis undergoing operative valve replacement or percutaneous transcatheter intervention with clinical, echocardiographic, and hemodynamic factors and prognosis. Am J Cardiol 112:574–579
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA et al (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardiogr 18:1440–1463
Iwataki M, Takeuchi M, Otani K, Kuwaki H, Haruki N, Yoshitani H, Tamura M, Abe H, Otsuji Y (2012) Measurement of left atrial volume from transthoracic three-dimensional echocardiographic data-sets using the biplane Simpson’s technique. J Am Soc Echocardiogr 25:1319–1326
Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR (2012) Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart 98:813–820
Acknowledgements
We thank Leah Cannon, PhD, from Edanz (https://www.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MO, ATK, RY, YK, TY, YT and TO. The first draft of the manuscript was written by AI and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Osaka City University Graduate School of Medicine (Approval Number: 2021-064).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ito, A., Izumiya, Y., Iwata, S. et al. Left atrial volume index predicts future improvement of B-type natriuretic peptide levels after transcatheter aortic valve replacement. Int J Cardiovasc Imaging 38, 1463–1471 (2022). https://doi.org/10.1007/s10554-022-02538-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-022-02538-7