Abstract
The aim of this study is to identify the best predictors of mortality among clinical, biochemical and advanced echocardiographic parameters in acute heart failure (AHF) patients admitted to coronary care unit (CCU). AHF is a clinical condition characterized by high mortality and morbidity. Several studies have investigated the potential prognostic factors that could help the risk assessment of cardiovascular events in HF patients, but at the moment it has not been found a complete prognostic score (including clinical, laboratory and echocardiographic parameters), univocally used for AHF patients. Patients (n = 118) admitted to CCU due to AHF de novo or to an exacerbation of chronic heart failure were enrolled. For each patient, clinical and biochemical parameters were reported as well as the echocardiographic data, including speckle tracking echocardiography analysis. These indexes were then related to intra- and extrahospital mortality. At the end of the follow-up period, the study population was divided into two groups, defined as ‘survivors’ and ‘non-survivors’. From statistical analysis, C-reactive protein (CRP) (AUC = 0.75), haemoglobin (AUC = 0.71), creatinine clearance (AUC = 0.74), left atrial strain (AUC = 0.73) and freewall right ventricular strain (AUC = 0.76) showed the strongest association with shortterm mortality and they represented the items of the proposed risk score, whose cut-off of 3 points is able to discriminate patients at higher risk of mortality. AHF represents one of the major challenges in CCU. The use of a combined biochemical and advanced echocardiographic score, assessed at admission, could help to better predict mortality risk, in addition to commonly used indexes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute heart failure (AHF) is a clinical condition defined as a rapid onset or a rapid worsening of typical signs and symptoms of heart failure (HF) [1]. Several studies have investigated the potential prognostic factors that could help the risk assessment of cardiovascular events in HF patients, but at the moment it has not been found a complete prognostic score (including anamnestic, clinical, laboratory and echocardiographic parameters), univocally used for AHF patients. In recent years, various risk scores have been outlined and applied to this subset of patients, but they clearly show some drawbacks, limiting their clinical application [2,3,4]. Two examples of the most used scores are the recent AHEAD score which predicts long-term risk in AHF [2] and Get With the Guidelines-Heart Failure (GWTG-HF) risk score used to predict in-hospital mortality, independently from left ventricular ejection fraction (LVEF) [3]. However, the majority of these scores lacks echocardiographic indexes or includes only LVEF, leaving out more recent and advanced parameters. For instance, LVEF strongly relies upon geometrical assumptions and it is greatly influenced by load conditions, which might make it a less reliable parameter in the acute setting. These pitfalls might be overcome by advanced echocardiographic techniques that have been developed over the years, such as speckle tracking echocardiography, which allows the evaluation of global and regional cardiac function, independently from the angle of insonation, making it suitable to be applied at the patient’s bed-side [4, 5].
The aim of this prospective study was to identify the best predictors of mortality and elaborate a prognostic score combining clinical, biochemical and echocardiographic parameters. In fact, the primary objective was to identify some parameters that could, already at the moment of hospitalization, be fairly easily related to the patient’s short-term prognosis, to optimize healthcare resources and to customize the therapeutic approach considering the severity of each patient.
Methods
Study design
This was an observational, prospective, single-centre study, developed in the coronary care unit (CCU) of the Department of Cardiovascular Diseases at University of Siena.
Patients older than 18 years-old, admitted to our CCU for de novo AHF, fulfilling the latest guidelines criteria [1], were eligible for inclusion. The presence of active cancer and poor echocardiographic window represented the exclusion criteria. All subjects gave their written informed consent to participate in the study.
Study population
From January 1st 2018 to March 31st 2019, a total of 146 patients were initially screened. Due to poor echocardiographic window 19 patients were excluded and other 9 patients because of the presence of neoplastic disease. A total of 118 patients were finally enrolled in the study. Figure 1 shows a study flow chart.
The primary endpoint was to find the strongest predictors of mortality among clinical, laboratory and echocardiographic indexes in patients admitted for AHF. Besides the patients that died in CCU, the others were enrolled in a follow-up in order to detect extra-hospital mortality. At the end of the follow-up period, the study population was divided into two groups, defined as ‘survivors’ (90 patients) and ‘non-survivors’ (28 patients). The mean follow-up period was 244 days.
For each patient, clinical, biochemical and echocardiographic parameters, in addition to the underlying cause of AHF itself, were collected within the first 24 h from admission in CCU. The maximum daily dose of intravenous diuretic and the duration of endovenous inotropic support was obtained from medical records at the end of CCU stay.
A thorough anamnesis was collected for each patient, including the age, sex, body surface index, body mass index and the presence of cardiovascular risk factors or intracardiac devices. Furthermore, vital signs and the presence of pulmonary congestion, evaluated through chest X-rays, as well as the need for intra-aortic balloon pump in the acute phase were reported.
Among biochemical parameters, indexes of systemic inflammation as erythrocyte sedimentation rate and C-reactive protein (CRP), creatinine, uric acid, haemoglobin, white blood cells, N-terminal pro-brain natriuretic peptide (NT-proBNP) and indexes of myocardial injury were recorded. Creatinine clearance was estimated employing Chronic Kidney Disease Epidemiology Collaboration formula.
Standard echocardiography
Transthoracic echocardiographic examinations were performed using a high quality echocardiograph (Vivid E9, GE, USA) equipped with a 3Mhz transducer. Subjects were studied in their left lateral recumbent position.
Standard echocardiographic measures included: left ventricular (LV) end-diastolic and end-systolic diameters from the parasternal long-axis, LV end-diastolic and end-systolic volumes, LVEF calculated by Simpson’s method, peak early (E) and late (A) diastolic transmitral flow velocity and E/A ratio, pulsed wave Tissue Doppler systolic (S’) and diastolic (E’, A’) velocities at both septal and lateral mitral annulus, E/E′ ratio as an estimate of LV filling pressure. Left atrial (LA) area and volume were assessed. LA volume was measured using the area–length method from apical 4- and 2-chamber views, and it was subsequently indexed for BSA. The evaluation of the right ventricle (RV) included: mid-end-diastolic diameter obtained from an apical 4-chamber view as dimensional parameter; tricuspidal anular plane systolic excursion (TAPSE), by M-mode technique, and pulsed wave Tissue Doppler systolic (s′) velocity at lateral tricuspid annulus, as standard echocardiographic indexes of longitudinal function; RV fractional area change (RVFAC) in 4-chamber view as index of global RV systolic function. Furthermore, right atrio-ventricular gradient was measured by continuous Doppler technique in presence of tricuspid regurgitation. Systolic pulmonary arterial pressure was estimated through the sum of the right atrio-ventricular gradient and the right atrial pressure, estimated by the dimensions and collapsibility of the inferior vena cava. Valvular regurgitation and/or stenosis and their severity were reported.
Speckle tracking echocardiography
Apical 4-, and 2-chamber views 2-dimensional grey scale echocardiographic images were collected, during a brief breath hold and with a stable electrocardiographic recording. Speckle Tracking analysis of the recorded images was then performed off-line by an independent and expert echocardiographer, not directly involved in image acquisition. Commercially available semiautomated two-dimensional strain software (EchoPac, Ge, Milwaukee, WI, USA) was used. Both ventricles and LA longitudinal deformations were evaluated.
The endocardial border was manually traced in each apical view, thus delineating a region of interest composed by three segments for LA and two segments for both ventricles. Then, after segmental tracking quality analysis and eventual manual adjustment of the region of interest, longitudinal strain curves for each segment were generated. LV global longitudinal strain (LV-GLS) was calculated by averaging the values observed in all LV segments in 4, 3 and 2-chamber views. Time to peak longitudinal stain was also measured as the average of all segments (global time to peak longitudinal strain) in the 4, 3 and 2 apical views.
Peak atrial longitudinal strain (PALS) of LA was calculated by averaging the values observed in all LA segments in 4- and 2-chamber views. Time to peak longitudinal strain was also measured as the average of all segments (global time to peak longitudinal strain) in the apical views.
From the 4-chamber apical image, RV global longitudinal strain (RV-GLS) and RV free-wall longitudinal strain (RVFWSL) were obtained averaging the values observed in all RV segments. Time to peak longitudinal strain was also measured as the average of all segments (global time to peak longitudinal strain) in the 4-apical view.
In presence of unsuitable segments due to the impossibility of achieving adequate tracking, RV, LV and LA longitudinal strain were calculated by averaging the values measured in the remaining segments.
For each chamber, it was also measured the maximum opposing wall delay, a parameter that represents the delay between peak radial strain of opposing walls of each chamber.
Statistical analysis
Regarding univariate correlations, the endpoint of interest was independently tested against each numeric and binary predictor via Correlation test. Correlation and respective p values are reported.
Due to the potential multiplicity problem caused by the high number of tests, Bonferroni correction was used when assessing the significance of the aforementioned p values. For the considered endpoint, highly correlated variables according to the univariate analysis were selected for inclusion in a multivariate analysis. Furthermore, a generalized linear model with binomial response and logit link function was fit.
In addition to univariate significance (as mentioned above), the number of variables included in the model was influenced by a qualitative assessment of potential data overfit and on the quantity of missing values.
A predictive score of death was calculated. Highly correlated and medically relevant predictive variables were selected. The selection of predictors took into account both univariate and multivariate analyses, correlations between predictors, and quantity of missing values.
A roc curve analysis (ROC)/area under the curve (AUC) assessment of the predictive value of each of the selected variables was performed. This assessment resulted in ideal cut-off points for each predictor. The cut-off points were used to discretize each predictor into binary variables. A logistic model (generalized linear model with binomial response and logit link) was used (with death as response) to estimate the relative weight of each discretized predictor. The relative weights were rounded to simplify score computation, resulting in a rounded weight of 2 for RVFWSL and of 1 for the other parameters. Then, a ROC/AUC assessment of the predictive score was performed.
Results
Clinical and echocardiographic characteristics of the study population
Table 1 summarizes both clinical and echocardiographic characteristics of the study population, divided into two groups ‘non-survivors’ and ‘survivors’. Over a mean follow-up period of 244 days, among the 118 enrolled patients, 28 patients died due to cardiovascular causes and between the 90 survivors, 18 have been re-hospitalized. Enrolled patients were admitted for: ischemic cardiomiopathy (62%), dilated cardiomiopathy (20%), heart valve disease (7%) and other causes (11%).
Systolic blood pressure was lower in non-survivors compared to survivors, whereas NT-pro-BNP values were more than double in non-survivors (p = 0.0013). Among the other biochemical parameters, mostly creatinine and creatinine clearance, troponin and also CRP, transaminase and Hb showed significant differences.
Regarding standard echocardiographic parameters, the first group had a lower LVEF, higher LA volume and estimated sPAP. Between STE parameters, significant differences were present for PALS (10.61 ± 7.4% in non-survivors vs 20.4 ± 14.3% in survivors, p = 0.0007) and RVFWSL (− 7.33 ± 5.08% in non-survivors vs − 13.04 ± 5.24% in survivors, p = 0.00001) (Fig. 2). On the other hand, LV-GLS did not significantly differ between the two groups (p = 0.1670).
Left atrial longitudinal strain and right ventricular free-wall longitudinal strain. On the left side of the figure, there are shown peak atrial longitudinal strain (PALS, at the top) and right ventricular free-wall longitudinal strain (RVFWSL, at the bottom) in a non-survivor patient, whereas, on the right side of the figure, PALS and RVFWSL are representative of a survivor patient
Predictors of mortality and risk score
ROC analysis showed an AUC between 0.71 and 0.76: CRP (AUC = 0.75), Hb (AUC = 0.71), creatinine clearance (AUC = 0.74), PALS (AUC = 0.73) and RVFWSL (AUC = 0.76) (Fig. 3). For each index a cut-off value was obtained, except from RVFWSL for which two cut-off values were identified. The five parameters were then combined in a prediction score in which 0 or 1 point were attributed to each variable according to its cut off, except from RVFWSL for which 1 or 2 points were added based on the two cut-off values (Table 2). A score > 3 predicted high mortality risk at short-term follow-up.
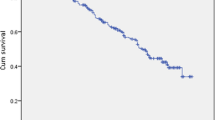
Kaplan Maier event-free survival curves (Fig. 4) demonstrate that patients with a score > 3 had a 40% probability of survival at 250 days, compared to 90% probability of those with a score lower than the proposed cut-off. The score AUC was 0.90, higher than the AUC of the single parameters.
Discussion
In this study we assessed which parameters, including clinical, laboratory and echocardiographic indexes, could possibly help in the prognostic stratification of patients admitted for AHF, considering both intra and extra-hospital mortality. Furthermore, we attempted to define the best predictors of outcome within a year from the first acute event through a short-term follow-up. From the results of our analysis, it emerged that several parameters have proved to be independent predictors of short-term mortality, among which CRP, haemoglobin, creatinine clearance, PALS and RVFWSL resulted to have the highest diagnostic accuracy and were therefore included in a multiparametric risk score with high sensitivity and specificity for short-term prognosis of these patients.
Biochemical parameters
Analysing the laboratory parameters most strongly correlated with mortality, it can be stated that CRP, a non-specific index of systemic phlogosis that through the production of pro-inflammatory cytokines contributes to ventricular remodeling and systolic and/or diastolic dysfunction, plays a leading role in prognosis of AHF. It is conceivable that the increase in this protein observed in the most critically ill patients reflects the degree of systemic impairment. The increase in CRP is also closely related to the presence of numerous comorbidities such as diabetes, chronic obstructive pulmonary disease, renal failure, peripheral artery disease, which contribute to the progression of HF, negatively impacting the prognosis of these patients. Over the years, it has been demonstrated that CRP represents a risk factor for cardiovascular diseases. High CRP plasma levels are associated with an increased risk of coronary artery disease and acute myocardial infarction [6, 7], also in agreement with what we found in our study population which was composed for 62% from patients with ischemic heart disease. A recent study also demonstrates how plasma concentrations of CRP correlate in a linear way with the values of NT-proBNP and with LVEF, highlighting a close association between CRP and HF [8].
Considering the other two laboratory parameters of our risk score, the role of creatinine clearance, regarded as an estimation of glomerular filtration rate, and haemoglobin as predictors of mortality, is widely recognized and there are numerous validated risk scores using these two markers, alone or in association. Hemoglobin and creatinine clearance could be predictors of mortality as an expression of the “cardiorenal syndrome” which very often complicates HF, leading to a worsening of renal function due to either hypoperfusion caused by reduced cardiac output or renal venous hypertension due to systemic congestion. Renal function impairment would therefore result on one hand in a reduction of glomerular filtration rate and on the other hand in a reduced synthesis of erythropoietin which would contribute to the typical anaemic state of the decompensated patient, triggering a dangerous vicious circle. In fact, acute renal failure and anaemia represent two important and serious complications that if not recognized and promptly treated, will lead to a significant worsening of the prognosis.
It was quite unexpected not to find NT-proBNP included as an item in the prognostic score. However, to a close analysis, our results are in line with the ones reported in literature. In fact, patients with higher levels of NT-proBNP at admission tended to have a worse prognosis [9]. However, it has been recently gaining importance NT-proBNP trend during hospitalization rather than the single value at admission [10]. In fact, a decrease of NT-proBNP levels from admission to discharge might hint a certain degree of response to treatment. However, in our study, we assessed only the admission levels, which might explain why it did not show a prognostic value as strong as haemoglobin, creatinine clearance and CRP at multivariate analysis. Another important prognostic marker in AHF is cardiac troponin [11], in fact several studies have shown that higher levels at admission carry a significant prognostic significance during follow-up. In AHF, high LV filling pressure and low cardiac output can induce a subtle subendocardial ischaemia due to worsening of the coronary perfusion gradient. Based on our results, troponin levels at admission significantly differed between survivors and non-survivors, even though multivariate analysis did not confirm their prognostic value.
Echocardiographic parameters
In the elaboration of the Risk Score, we also considered the impairment of cardiac function, analysed both by conventional echocardiography and by speckle tracking echocardiography. Using the latter method, two parameters proved to be the best predictors of mortality: PALS and RVFWSL. In fact, important differences were found between LA and RV strain values of survivors and non survivors, despite the overall values of the population being generally lower than the reference values.
The evaluation of LA function is of paramount importance in patients with HF fulfilling both a diagnostic role, in the context of symptomatic diastolic dysfunction, and a prognostic one, since it represents an index of increase in LV filling pressures [12], acting as an early and non-invasive marker of the disease [13, 14]. In a recent study [15], it was shown that in patients with HF and preserved LVEF parameters such as indexed LA volume and the E/e′ ratio increase with the degree of diastolic dysfunction but loose significance in the more severe patterns of diastolic dysfunction (pseudonormal and restrictive pattern) failing to discriminate between them. PALS instead has shown a progressive and constant reduction as the degree of diastolic dysfunction progresses, proving to be the most reliable parameter for the evaluation of LA function [16].
In both types of HF, LA impairment can be associated with RV dysfunction and pulmonary hypertension as well as with perceived quality of life [17], but above all, in the subgroup with preserved LVEF it has shown to have a close correlation with the patients’ outcome [18], highlighting the importance of improving LA with appropriate therapy. Sanchis et al. showed that the strain of the contractile phase of the LA represents a predictor of cardiovascular outcome even in outpatients with HF [19]. In their study, patients with worse atrial function had a worse event-free survival, regardless of LVEF, thus stressing the important role of LA function in the heart failure syndrome. Other recent studies demonstrated also the association between low PALS values and increased risk of hospitalization for heart failure, independently of other clinical factors, as well as the negative correlation between PALS and NYHA class [20, 21]. The superiority of PALS in predicting short-term mortality in patients with AHF was therefore confirmed in our study as compared to conventional echocardiographic measurement, such as the indexed left atrial volume.
Regarding the study of RV function, the validated parameters traditionally used in patients with HF have strong limitations both for the dependence on preload conditions and the angle of insonation, and for the complex morphology of the RV [22, 23]. RV dysfunction may not simply be the consequence of LV dysfunction but it might represent a pathophysiological event that accompanies LV failure, involving the concept of ventricular interdependence in a common pathological process that affects both sections of the heart [24]. RV can be indeed dysfunctional both due to an increase in pulmonary pressures caused by a reduced LV function and because it may be affected by the same pathological process that affects LV. In another study, it was observed that both RVFWSL and indexed LA volume represent independent predictors of cardiovascular events in HF with reduced LVEF and in particular RVFWSL resulted to be an independent predictor even in AHF and in HF with preserved LVEF, whereas neither RV-GLS nor LV-GLS were [25]. This is probably explained by the fact that the RV-GLS is influenced by LV function due to the important contribution of the interventricular septum, which instead is not considered when RVFWSL is assessed instead. Other studies have confirmed the prognostic role of RV dysfunction in HF [26, 27], independently of LV function [28, 29], as well as in various cardiovascular diseases [30,31,32]. The use of STE methods in the study of RV function and in particular RVFWSL also has two important clinical applications: it is in fact an excellent predictor of outcome in patients with advanced HF awaiting cardiac transplantation [33] and represents an effective tool in the selection of candidates for the implantation of left ventricular assist devices (LVAD), identifying those most likely to experience acute post-implant RV failure, an event burdened by high rates mortality and morbidity, as well as significant health costs.
Study limitation
Regarding the limits of the study, the main one is represented by the small population, therefore the score will need a validation on a larger sample. Secondly, the measurement of LA and RV strain requires adequate apical views and experience for a more reliable delineation of the endocardial border and for the analysis. Considering the analysed biochemical parameters, this study lacks the data derived from newer biomarkers, such as suppression of tumorigenicity 2 (ST2). Furthermore, it has not been possible to assess lactate level and central oxygen saturation in every patient, since in cases of mild decompensation of HF our CCU protocol does not advise the placement of a central venous catheter.
Conclusions
To the best of our knowledge, this is the first prospective study that proposed a predictive score of mortality and short-term outcome in patients presenting with AHF, including biochemical and advanced echocardiographic parameters. Our risk score is composed of five elements, such as CRP, haemoglobin, creatinine clearance, PALS and RVFWSL. The proposed risk score, highly feasible even in intensive care unit, could provide a rapid assessment of the prognosis of patients presenting with AHF and help the clinician to adopt the optimal therapeutic strategy.
References
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG et al (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur J Heart Failure 18(8):891–975
Felker GM, Leimberger JD, Califf RM, Cuffe MS et al (2004) Risk stratification after hospitalization for decompensated heart failure. J Cardiol Fail 10(6):460–466
Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV (2003) Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA 290(19):2581–2587
Uszko-Lencer NHMK, Frankenstein L, Spruit MA et al (2017) Predicting hospitalization and mortality in patients with heart failure: the BARDICHE-index. Int J Cardiol 227:901–907
Kocher RP, Adashi EY (2011) Hospital readmissions and the Affordable Care Act: paying for coordinated quality care. JAMA 306(16):1794–1795
Emerging Risk Factors Collaboration, Kaptoge S, Di-Angelantonio E, Lowe G et al (2010) C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 375(9709):132–140
St-Pierre AC, Cantin B, Mauriège P et al (2005) Insulin resistance syndrome, body mass index and the risk of ischemic heart disease. CMAJ 172(10):1301–1305
Kang S, Fan LY, Chen M, Li J, Liu ZM (2017) Relationship of high-sensitivity C-reactive protein concentrations and systolic heart failure. Curr Vasc Pharmacol 15(4):390–396
Avellino A, Collins SP, Fermann GJ (2011) Risk stratification and short-term prognosis in acute heart failure syndromes: a review of novel biomarkers. Biomarkers 16:379–392
Santaguida PL, Don-Wauchope AC, Oremus M, McKelvie R, Ali U, Hill SA, Balion C, Booth RA, Brown JA, Bustamam A, Sohel N, Raina P (2014) BNP and NT-proBNP as prognostic markers in persons with acute decompensated heart failure: a systematic review. Heart Fail Rev 19(4):453–470
Harrison N, Favot M, Levy P (2019) The role of troponin for acute heart failure. Curr Heart Fail Rep 16(1):21–31
Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB (2002) Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 90(12):1284–1289
Santos AB, Kraigher-Krainer E, Gupta DK, Claggett B, Zile MR et al (2014) Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail 16(10):1096–1103
Cameli M, Sparla S, Losito M, Righini FM et al (2016) Correlation of left atrial strain and doppler measurements with invasive measurement of left ventricular end-diastolic pressure in patients stratified for different values of ejection fraction. Echocardiography 33(3):398–405
Singh A, Addetia K, Maffessanti F, Mor-Avi V, Lang RM (2017) LA strain for categorization of LV diastolic dysfunction. JACC Cardiovasc Imaging 10(7):735–743
Nagueh SF, Appleton CP, Gillebert TC, Marino PN et al (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 10(2):165–193
Cameli M, Sciaccaluga C, Loiacono F, Simova I, Miglioranza MH, Nistor D, Bandera F, Emdin M, Giannoni A, Ciccone MM, Devito F, Guaricci AI, Favale S, Lisi M, Mandoli GE, Henein M, Mondillo S (2019) The analysis of left atrial function predicts the severity of functional impairment in chronic heart failure: the FLASH multicenter study. Int J Cardiol 286:87–91
Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA (2015) Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail 8(2):295–303
Sanchis L, Andrea R, Falces C, Lopez-Sobrino T, Montserrat S, Perez-Villa F, Bijnens B, Sitges M (2016) Prognostic value of left atrial strain in outpatients with de novo heart failure. J Am Soc Echocardiogr 29(11):1035-1042.e1
Santos AB, Roca GQ, Claggett B, Sweitzer NK et al (2016) Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail 9(4):e002763
Roşca M, Popescu BA, Beladan CC et al (2010) Left atrial dysfunction as a correlate of heart failure symptoms in hypertrophic cardiomyopathy. J Am Soc Echocardiogr 23(10):1090–1098
Cameli M, Loiacono F, Sparla S, Solari M et al (2017) Systematic left ventricular assist device implant eligibility with non-invasive assessment: the SIENA protocol. J Cardiovasc Ultrasound 25(2):39–46
Abraham J, Abraham TP (2009) The role of echocardiography in hemodynamic assessment in heart failure. Heart Fail Clin 5(2):191–208
Bosch L, Lam CSP, Gong L, Chan SP, Sim D, Yeo D, Jaufeerally F, Leong KTG, Ong HY, Ng TP, Richards AM, Arslan F, Ling LH (2017) Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail 19(12):1664–1671
Hamada-Harimura Y, Seo Y, Ishizu T, Nishi I, Machino-Ohtsuka T, Yamamoto M, Sugano A, Sato K, Sai S, Obara K, Yoshida I, Aonuma K, ICAS Investigators (2018) Incremental prognostic value of right ventricular strain in patients with acute decompensated heart failure. Circ Cardiovasc Imaging 11(10):e007249
De Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, Lablanche JM (1998) Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol 32:948–954
Di Salvo TG, Mathier M, Semigran MJ, Dec GW (1995) Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol 25:1143–1153
Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA (2014) Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 35:3452–3462
Mohammed SF, Hussain I, AbouEzzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM (2014) Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation 130:2310–2320
Park SJ, Park JH, Lee HS, Kim MS, Park YK, Park Y, Kim YJ, Lee JH, Choi SW, Jeong JO, Kwon IS, Seong IW (2015) Impaired RV global longitudinal strain is associated with poor long-term clinical outcomes in patients with acute inferior STEMI. JACC Cardiovasc Imaging 8:161–169
Fukuda Y, Tanaka H, Sugiyama D, Ryo K, Onishi T, Fukuya H, Nogami M, Ohno Y, Emoto N, Kawai H, Hirata K (2011) Utility of right ventricular free wall speckle-tracking strain for evaluation of right ventricular performance in patients with pulmonary hypertension. J Am Soc Echocardiogr 24:1101–1108
Park JH, Park MM, Farha S, Sharp J, Lundgrin E, Comhair S, Tang WH, Erzurum SC, Thomas JD (2015) Impaired global right ventricular longitudinal strain predicts long-term adverse outcomes in patients with pulmonary arterial hypertension. J Cardiovasc Ultrasound 23:91–99
Cameli M, Righini FM, Lisi M, Bennati E et al (2013) Comparison of right versus left ventricular strain analysis as a predictor of outcome in patients with systolic heart failure referred for heart transplantation. Am J Cardiol 112(11):1778–1784
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sciaccaluga, C., Mandoli, G.E., Nannelli, C. et al. Survival in acute heart failure in intensive cardiac care unit: a prospective study. Int J Cardiovasc Imaging 37, 1245–1253 (2021). https://doi.org/10.1007/s10554-020-02109-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-02109-8