Abstract
The success rate of percutaneous coronary artery intervention (PCI) of chronic total occlusion (CTO) lesions have increased in the recent years. However, improvement of function is only possible when significant myocardial viability is present. One of the most important factors of maintaining myocardial viability is the opening and development of collaterals. Our hypothesis was that with a higher degree of collaterals more viable myocardium is present. In 38 patients we compared the degree of collaterals, evaluated with a conventional coronary angiogram (CCA) and graded by the Rentrop classification to transmural extent of the scar obtained in a viability study with magnetic resonance (MRI). We found a statistically significant association of the degree of collaterals determined with Rentrop method and transmural extent of the scar as measured by CMR (p = 0.001; Tau = -0.144). Additionally, associations showed an increase in the ratio between viable vs. non-viable myocardium with the degree of collaterals. Our study suggests that it may be beneficial to routinely grade the collaterals at angiography in patients with CTO as an assessment of myocardial viability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the last decade, techniques for percutaneous coronary artery intervention (PCI) of chronic total occlusion (CTO) have developed significantly, leading to the success rate of 85–90% in hands of dedicated operators [1]. Although PCI of CTO does not reduce major adverse cardiovascular events (MACE), there may be significant improvement in the quality in life [2]. However, such benefits of PCI CTO are likely only if significant myocardial viability is present in the CTO territory. The evaluation of myocardial viability before PCI is therefore of interest.
Various factors may modify the natural course and size of myocardial infarction in the acute stage: duration of ischemia, size, and location of occlusion, coronary cardioprotective medication and coronary artery anatomy [3]. The extent and duration of reduced or total stoppage of blood flow and oxygen supply are determinants of partial (chronic) or total ischemia. The development and/or opening of pre-performed collateral coronary vasculature (arteriogenesis) are thought to be one of the very important factors limiting chronic ischemia [4], and through this keeping the myocardium viable [3,4,5,6,7,8,9].
Various clinical studies have demonstrated results in support of a close relationship between the presence of collateral coronary circulation and myocardial viability [10,11,12,13,14,15,16,17,18], whereas several other studies could not confirm this hypothesis [19, 20].
We addressed the relationship between extent of collateral supply in the CTO territory assessed with conventional coronary angiogram (CCA) and myocardial viability using magnetic resonance imaging (MRI), which is a gold standard for myocardial viability imaging, although different modalities are being used for the evaluation [21, 22].. The goal of this prospective study was to elucidate using sophisticated imaging methods the positive relationship between the presence of functioning coronary collateral circulation and the amount of viable myocardium in patients with CTO as defined by the consensus document from the Euro CTO club [23].
Methods
Study population
Forty-two patients were recruited for our study between 2011 and 2018 after an elective CCA revealed a CTO. Within 1 month of diagnosis all patients were studied by Cardiac Magnetic Resonance (CMR) using a standard protocol using intravenous contrast agent (Gadovist, Bayer). In conjunction with the patient’s informed consent form, patient history data was obtained using a questionnaire. Questions about smoking, diabetes, hypertension, statins therapy, a positive family history of coronary heart disease, regular exercise and angina pectoris were answered with a yes/no answer option. Late gadolinium enhanced images were obtained 10 min after contrast administration and used for evaluation of myocardial viability defined as less than 50% transmural enhancement. Two of the patients revealed late enhancement patterns suggestive of infiltrative cardiomyopathy. In two patients the re-evaluation of CCA could not confirm a CTO lesion. These four patients were excluded from further analysis with a final number of 38 patients enrolled in the study. The study was approved by the National ethics committee of Slovenia.
CCA
In the clinical setting CCA is used as gold standard for depicting coronary collaterals. For comparative angiographic assessment of visible coronary collateral vessels, Rentrop grading system was used [24, 25] (Table 1). The grading was performed by an experienced interventional cardiologist.
CMR
The CMR images were obtained using a 1,5 T scanner (Signa Excite, GE Waukesha, USA). The CMR protocol consisted of cine imaging steady-state free precession (SSFP) in two, four chamber and short axis views. A slice thickness of 8 mm with 2 mm spacing was used. Late gadolinium enhancement (LGE) images were obtained 10 min after contrast administration using a segmented gradient echo inversion recovery LGE sequence. Short axis views with a slice thickness of 8 mm without spacing were obtained. Long axis images were obtained in every of the 17 segments and additional images if delayed enhancement was present.
Left ventricular parameters as EDV, ESV, SV, EF, LVMM were determined using Medis Suite Cardiovascular MR software (the Netherlands). EDV, ESV, SV and CO have been indexed to body surface area (BSA). As the reference for normal values we took the EACVI consensus paper on normal values of cardiac chamber size ECS guidelines [26]. Since the patients were predominantly male (87%), we opted for the reference values of males. In addition, rate pressure product (RPP) and systemic vascular resistance (SVR) have been calculated. The end diastolic wall thickness (EDWT) was measured on cine images at the end of diastole and reported in millimeters and compared to normal values [27].
Gadolinium contrast agent was administered intravenously with a flow of 3,5 ml/s and a dose of 0,2 mmol/kg. Left ventricular wall enhancement 10 min after contrast agent administration was evaluated. Due to the interest of CTO, we analyzed only segments that were referred to the CTO vessel territory. Segmentation was based on the AHA 17-segment model. Two hundred and ninety of the total 684 segments were included into analysis.
Scar tissue was assessed by the presence of late gadolinium enhancement by the grading approached introduced by Kim [28]–(Table1). The scar tissue was measured in millimeters perpendicular to the subendocardial layer at maximum scar level in the segment and then divided by the thickness of the whole myocardial wall in this segment. The number obtained was multiplied by 100 and presented as percentage.
Statistical analysis
Data were analyzed using IBM SPSS software version 25.0 (IBM Inc., Armonk, New York) and R (R Core Team 2017, Vienna, Austria). Differences of continuous or ordinal variables between categorical nominal groups were determined using Mann–Whitney U-Test and Kruskal–Wallis H-Test with applied pairwise comparisons. Ordinal by continuous correlations and ordinal by ordinal associations were determined using Spearman’s rank and Kendall’s Tau b or c correlations, respectively. Differences between nominal categorical variables were determined using Chi-square statistics. A receiver operating characteristic (ROC) curve analysis was applied to determine area under curve (AUC) for predictors. Alpha level was set to 5% and p value < 0.05 was considered statistically significant.
Results
Patient's characteristics
Table 2 is showing essential patient's characteristics of 33 male and 5 female patients included in this study. 21 (55%) were smokers, 18 (47%) had diabetes type 2 and all of them were on glycemic control therapy, no patients had type 1 diabetes. 27 patients were treated for hypertension and 30 patients (79%) were on statins. Only 11 patients (28%) had a positive family history with a parent or sibling having a cardiac event under the age of 55 for males and 65 for females [29]. Twenty patients were taking regular time for physical activity referred by them as exercise (at least 30 min of walking or cycling). 13 patients (34%) were experiencing angina on exertion.
Volumetric parameters
The mean values of the ejection fraction in our patient selection were reduced by 26% of normal values accompanying by an increase of the LV end diastolic volume index of 31.6 ml/m2 from normal values. Rate pressure product was within low normal values. Systemic vascular resistance was at the upper normal level (Table 3).
Dominance
Only CTO related territories were evaluated using the AHA (American Heart Association) 17-segment model [28, 30]. We assigned 7 segments to the LAD territory, 5 to RCA and 5 to LCX territory in case of right dominating system. In case of a left dominating system, RCA had only 3 segments and LCX had 7. In total 290 CTO segments were evaluated. On average 7, 6 CTO segments were identified per patient.
Right dominant system was present in 34 patients, left dominant system in 2 patients and a codominant system also in 2 patients. In both codominant cases RCA was the CTO vessel. In one case the degree of collaterals was rated as Rentrop grade 3 and the other as Rentrop grade 1. We analyzed all the segments as being a CTO territory (Table 4).
Site of occlusion
Proximal coronary vessel occlusion was found in 86 segments and mid/vessel occlusion was found in 204 segments. No distal occlusion was seen.
Collateral grade
Collateral Grade 0 was found in 10 segments, Grade 1 in 31 segments, Grade 2 in 56 segments and Grade 3 in 123 segments (Table 5)
Transmural extent of the scar
Transmural extent of the scar of 1–25% (Grade 1) was present in 26 segments (8,9%), transmural extent of the scar of 26–50% (Grade 2) in 69 segments (23,8%), a transmural extent of the scar of 51–75% (Grade 3) was present in 33 segments (11,4%) and a transmural extent of the scar of 76–100% (Grade 4) in 24 segments (8,3%). 138 CTO segments (47%) were without a scar present (Grade 0) (Table 6)
Relationship between scar thickness and degree of collaterals
We found statistically significant difference of the percent of transmural scar extent between different Rentrop collateral grades (p = 0.016) (Fig. 1 a, b). With subsequent pairwise comparisons the results have shown statistically significant decrease of the transmural scar extent in the group with grade 3 collaterals (21.98 ± 29.16; median: 0) in comparison with grade 2 (30.89 ± 31.97; median 25; p = 0.013) or grade 1 (38.90 ± 34.81; median 42.33; p = 0.008) collaterals (Fig. 1 a). No statistically significant difference was observed in comparison of grade 1 and grade 2 collaterals. Group of grade 0 collaterals couldn’t be assessed due to low number of cases (N = 10). Additionally ordinal correlation confirmed a negative association between Rentrop collateral grade and ordinally classified transmural extent of the scar (p = 0.001; Tau = − 0.144).
In addition, scar thickness was classified into dichotomous groups below and including 50% and more than 50% to represent viable and non-viable myocardium, respectively. Our results show that the degree of collaterals is statistically significant higher in the group with scar thickness below and including 50% (mean 2.31 ± 0.78) as compared to the group with scar thickness above 50% (mean 1.98 ± 0.74; p = 0.001) (Fig. 2). The ratio between viable vs. non-viable myocardium was also increased with the degree of collaterals and was found to be significantly different (p = 0.006) between grades of collaterals (Fig. 3).
Influence of risk factors
Influence of risk factors was assessed using a questionnaire and clinical data of present weight, cholesterol, and diabetes status of enrolled patients. We found statistically significant difference of contractility level between patients with hypercholesterolemia, but no statistical difference was observed for degree of collaterals, transmural extent of the scar or EDWT. We found that patients with hypercholesterolemia exhibit lower level of contractility (mean 1.45 ± 1.06) in comparison with the patients without hypercholesterolemia (mean: 1.95 ± 0.83; p < 0.001). Regarding the presence of diabetes, we observed statistically significant lower level of transmural extent of the scar in patients with diabetes (mean: 1.00 ± 1.24) in comparison with the patients without diabetes (mean 1.48 ± 1.45; p = 0.007). EDTW was statistically significant higher in patients with diabetes (mean 7.06 ± 2.49) in comparison with the patients without diabetes (mean: 5.83 ± 2.53; p < -0.001), but no statistically significant difference was found for degree of collaterals and contractility. Transmural extent of the scar level was also statistically significant higher in patients who smoke (mean 1.53 ± 1.41) in comparison with non-smokers (mean 0.84 ± 1.23; p < 0.001), but no statistically significant difference was observed for degree of collaterals, contractility or EDTW. No statistically significant difference was found in relation with the aforementioned parameters and BMI.
Discussion
Our results confirm a positive relationship between the presence of functioning coronary collateral circulation and the amount of viable myocardium in patients with CTO. Figure 4 illustrates a case of insufficient collaterals with subsequent lower ejection fraction and a scar of > 75% of the anterior wall (Fig. 4). Figure 5 illustrates a case of well-developed left to right collaterals, a good ejection fraction and no fibrosis (Fig. 5).
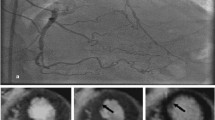
Patient A: (upper left) angiography showing a proximal CTO LAD (black arrow) with very weak auto-collaterals (blue arrow) (Rentrop 0–1) which fill mainly ramus intermedius and not mid-distal LAD (Rentrop 0–1). There were no right to left collaterals to LAD; (on the right) SSFP 2CH image in systole a and diastole b with an ejection fraction of 30% as well as no thickening of the myocardium in the anterior wall; (bottom row) delayed enhancement images in short axis view (apical, mid-ventricular, basal slice) show a more than 75% extend of the scar in segments 7,13 and 14. The 2CH long axis confirms the thin anterior wall in the mid and apical segments (bottom right). Additional subendocardial scar after an LCX infarct, that was spontaneously recanalized (green arrow)
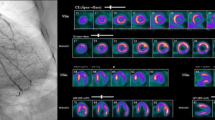
Patient B: (upper left) angiography showing a CTO RCA (black arrow) and (upper right); well-developed collaterals (Rentrop 3) from LAD to distal (PDA,PLV) and mid RCA (mid row left) SSFP images in systole and diastole (mid row right) with good ejection fraction (70%); (bottom row) delayed enhancement images in SA (apical, mid-ventricular, basal) and 2CH showing no fibrosis
Our study confirms that with the increasing degree of collaterals, the amount of viable myocardium increases. The inverse correlation is supported by an increasing ratio between viable and non-viable myocardium which we used to decrease the bias due to different sample sizes amongst the collateral groups.
Our results are in concordance with the study performed by Ripley et al. [17] and Choi et al. [18], but not in concordance with the recent study by Wang et al. [19]. Unlike our study, Wang el al did not find a correlation between the degree of collaterals and amount of viable myocardium. This may be due to our inclusion criteria being the same as for the Choi’s study whereas the Wang study excluded patients with a preserved systolic function (EF%). Preserved EF is, from our point of view, a clinical feature of well-developed collateral system and a consequence of preserved contractility.
The degree of collateralization was recently reported to be predictive of procedural success of CTO-PCI in a recent study by Allahvala et al. [31] and Schumacher et al. [32] in support of our observations.
In a previous study He et al. [20] attempted to avoid other parameters that might influence collateral development and myocardial perfusion by excluding patients with multi vessel disease, severe stenosis, or previous myocardial infarction. We only excluded patients for which a CTO could not be confirmed. We also did not exclude patients who had two CTO lesions or severe atherosclerosis. There is a possibility that coronary steal from the CTO territory affects the remote myocardium and contributes to lower ejection fraction. However, we therefore only focused on the correlation of transmural extend of the scar and the degree of collaterals, exclusively in a CTO territory. The non-CTO territories were not evaluated. In He’s study the degree of collaterals was not graded therefore a correlation was not evaluated. However, they did confirm that collateral circulation influences viability and the lack of collateral circulation does not exclude viability. The latter was also our finding, since despite a strong overall correlation among contractility and transmural extent of the scar with the collateral degree, we also had a few viable segments in the group where no collaterals were present. The number of these segments was too low to make a statement in the statistical analyses. However, from a clinical point of view, the reason for having viable myocardium with no visible collaterals might be the limit to the size of the vessel we can depict (> 100 µm) [33]. Also, a human error while measuring the extent of the scar with values around 50% is possible.
On the other hand, some segments with transmural extent of the scar of > 50% (non-viable) were present in the groups with the 2 or 3 grades of collaterals. These segments were studied in a territory manner/on a patient basis. For example, if the segment was evaluated by MR as not viable, but the collateral vessel grading for the assigned CTO lesion as Grade 2 or 3, we looked at all segments that part of the CTO territory. The evaluated segments were assigned to 17 patients. Nine of those patients had at least 70% and one had 60% of viable segments within the affected vessel territory. The presence of nonviable segments within a viable territory could be explained with the downregulation of the collateral development after the sheer stress pressure falls to normal. By this the main trigger for arteriogenesis is not present and the collateral development stops [33,34,35,36,37,38,39]. Another reason could be that the reference standard of transmurality is imperfect, as shown in a study by Nagel [40]. All nine patients with segments where the transmural extent of the scar was > 50% and had collateral grade of 2 or 3 were male, but no correlation with other factors was found.
Four of the remaining patients with good collaterals but transmural extent of the scar > 50% were smokers. Three were male and one patient was female. In our study the only significant difference of smoking as a risk factor was with transmural extent of the scar. Patients who smoked had an increase in transmural extent of the scar than non-smokers. The effects of smoking [41] and gender [42] on the microcirculation have been well-documented.
By analyzing influence of risk factors in addition to smoking we found a difference in contractility in patients who were on statins. Those on statin therapy had less wall motion abnormalities. The positive effect of statins on the endothelium has been previously documented [43]. The important difference in patients on glycemic control therapy was that their EDWT was higher and the scar was thinner than in the group without diabetes, respectively. The effects on LV mass in diabetic people has also been confirmed by other studies [44]. Whether reduced infarct size is a consequence of glycemic control therapy or better collateral development remains unclear in our study.
Finally, we looked at the site of occlusion. There was a suggestion of better collateral development in the case of a mid-vessel occlusion when compared with a proximal occlusion. None of our patients had a distal occlusion.
Our study was a single center retrospective study and it is therefore exploratory. Recovery of function following opening the CTO would be the best parameter to support our conclusions, but no MR follow-up study to evaluate the improvement after opening the CTO lesion was made. It has been shown that opening a CTO lesion improves the quality of life and relieves symptoms [2]. It would also be interesting to see, whether the improvement is greater with a higher degree of collaterals. The relatively low number of patients is another limitation to the study. With the increasing number there might also be a significant difference between the site of occlusion and degree of collaterals, which would be also interesting to see in future studies. Another limitation to our study is Rentrop grading being assessed by one interventional cardiologist, therefore reproducibility remains questionable.
Conclusion
Different imaging modalities use different parameters to evaluate for viability. Echocardiography enables us to evaluate contractility, EDWT as well as volumetric analysis CMR contractility, EDWT and transmural extent of the scar (also perfusion and stress perfusion) in addition to volumetric analyses, whereas angiography is limited by 2D views to contractility and depiction of collaterals. As for now the best evaluation of viability is obtained with a MR study, but not every center has an MR available. Our study suggests that there is a correlation between collaterals at angiography in patients with CTO and myocardial viability but the importance of this observation requires follow-up data. In the case of a CTO lesion with good collaterals (Rentrop Garde 2 or 3) this information could guide the decision making on opening a CTO lesion.
References
Galassi AR, Werner GS, Boukhris M, Azzalini L, Mashayekhi K, Carlino M, Avran A, Konstantinidis NV, Grancini L, Bryniarski L, Garbo R, Bozinovic N, Gershlick AH, Rathore S, Di Mario C, Louvard Y, Reifart N, Sianos G (2019) On behalf of the EuroCTO club. EuroIntervention 15:198–208
Werner GS, Martin-Yuste V, Hildick-Smith D, Boudou N, Sianos G, Gelev V, Rumoroso JR, Erglis A, Christiansen EH, Escaned J, di Mario C, Hovasse T, Teruel L, Bufe A, Lauer B, Bogaerts K, Goicolea J, Spratt JC, Gershlick AH, Galassi AR, Louvard Y, EUROCTO trial investigators (2018) A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J 39(26):2484–2493
Gibson CM, Schömig A (2004) Coronary and myocardial angiography: angiographic assessment of both epicardial and myocardial perfusion. Circulation 109(25):3096–3105
Seiler C (2010) The human coronary collateral circulation. Eur J Clin Invest 40(5):465–476
Vanoverschelde JL, Wijns W, Depré C, Essamri B, Heyndrickx GR, Borgers M et al (1993) Mechanisms of chronic regional postischemic dysfunction in humans. New insights from the study of noninfarcted collateral-dependent myocardium. Circulation 87(5):1513–1523
van de Hoef TP, Bax M, Damman P, Delewi R, Hassell ME, Piek MA, Chamuleau SA, Voskuil M, van Eck-Smit BL, Verberne HJ, Henriques JP, Koch KT, de Winter RJ, Tijssen JG, Piek JJ, Meuwissen M (2013) Impaired coronary autoregulation is associated with long-term fatal events in patients with stable coronary artery disease. Circ Cardiovasc Interv 6:329–335
Camici PG, d’Amati G, Rimoldi O (2015) Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol 12:48–62
Crea F, Camici PG, Bairey Merz CN (2014) Coronary microvascular dysfunction: an update. Eur Heart J 35:1101–1111
Choo G-H (2015) Collateral circulation in chronic total occlusions‐an interventional perspective. Curr Cardiol Rev 11(4):277–284
Fukai M, Ii M, Nakakoji T, Kawakatsu M, Nariyama J, Yokota N et al (2000) Angiographically demonstrated coronary collaterals predict residual viable myocardium in patients with chronic myocardial infarction: a regional metabolic study. J Cardiol 35(2):103–111
Ortiz-Pérez JT, Meyers SN, Lee DC, Kansal P, Klocke FJ, Holly TA et al (2007) Angiographic estimates of myocardium at risk during acute myocardial infarction: validation study using cardiac magnetic resonance imaging. Eur Heart J 28(14):1750–1758
Desch S, de Waha S, Eitel I, Koch A, Gutberlet M, Schuler G et al (2010) Effect of coronary collaterals on long-term prognosis in patients undergoing primary angioplasty for acute ST-elevation myocardial infarction. Am J Cardiol 106(5):605–611
Nicolau JC, Pinto MA, Nogueira PR, Lorga AM, Jacob JL, Garzon SA (1997) The role of antegrade and collateral flow in relation to left ventricular function post-thrombolysis. Int J Cardiol 61(1):47–54
Elsman P, van 'tHof AW, de Boer MJ, Hoorntje JC, Suryapranata H, Dambrink JH et al (2004) Role of collateral circulation in the acute phase of ST-segment-elevation myocardial infarction treated with primary coronary intervention. Eur Heart J 25(10):854–858
Nakatani D, Sato H, Kinjo K, Mizuno H, Hishida E, Hirayama A et al (2003) Effect of successful late reperfusion by primary coronary angioplasty on mechanical complications of acute myocardial infarction. Am J Cardiol 92(7):785–788
Pérez-Castellano N, García EJ, Abeytua M, Soriano J, Serrano JA, Elízaga J et al (1998) Influence of collateral circulation on in-hospital death from anterior acute myocardial infarction. J Am Coll Cardiol 31(3):512–518
Ripley DP, Gosling OE, Bhatia L, Peebles CR, Shore AC, Curzen N et al (2014) The relationship between the contralateral collateral supply and myocardial viability on cardiovascular magnetic resonance: can the angiogram predict functional recovery? Int J Cardiol 177(2):362–367
Choi JH, Chang SA, Choi JO, Song YB, Hahn JY, Choi SH et al (2013) Frequency of myocardial infarction and its relationship to angiographic collateral flow in territories supplied by chronically occluded coronary arteries. Circulation 127:703–709
Wang L, Lu MJ, Feng L, Wang J, Fang W, He ZX et al (2019) Relationship of myocardial hibernation, scar, and angiographic collateral flow in ischemic cardiomyopathy with coronary chronic total occlusion. J Nucl Cardiol 26(5):1720–1730
He ZX, Mahmarian JJ, Verani MS (2001) Myocardial perfusion in patients with total occlusion of a single coronary artery with and without collateral circulation. J Nucl Cardiol 8:452–457
Löffler AI, Kramer CM (2018) Myocardial viability testing to guide coronary revascularization. Interv Cardiol Clin 7(3):355–365
Krajnc I, Sinkovič A (2019) Assessment of left ventricular impairment by calculating left ventricular impairment index using doppler echocardiography in chronic heart failure patients. Acta Medico-Biotechnica 12(2):39–48
Di Mario C, Werner GS, Sianos G, Galassi AR, Büttner J, Dudek D, Chevalier B, Lefevre T, Schofer J, Koolen J, Sievert H, Reimers B, Fajadet J, Colombo A, Gershlick A, Serruys PW, Reifart N (2007) European perspective in the recanalisation of chronic total occlusions (CTO): consensus document from the EuroCTO club. EuroIntervention 3(1):30–43
Werner GS, Ferrari M, Heinke S, Kuethe F, Surber R, Richartz BM et al (2003) Angiographic assessment of collateral connections in comparison with invasively determined collateral function in chronic coronary occlusions. Circulation 107(15):1972–1977
Rentrop KP, Cohen M, Blanke H, Phillips RA (1985) Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 5(3):587–592
Petersen SE, Khanji MY, Plein S, Lancellotti P, Chiara Bucciarelli-Ducci (2019) European association of cardiovascular imaging expert consensus paper: a comprehensive review of cardiovascular magnetic resonance normal values of cardiac chamber size and aortic root in adults and recommendations for grading severity. Eur Heart J Cardiovasc Imaging 20(12):1321–1331
Kawel N, Turkbey EB, Carr JJ, Eng J, Gomes AS, Hundley WG, Johnson C, Masri SC, Prince MR, van der Geest RJ, Lima JCA, Bluemke DA (2012) Normal left ventricular myocardial thickness for middle aged and older subjects with SSFP cardiac MR: the multi-ethnic study of Atherosclerosis. Circ Cardiovasc Imaging 5(4):500–508
Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O et al (2000) The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 343(20):1445–1453
Allport SA, Kikah N, Saif NA, Ekokobe F, Atem FD (2016) Parental age of onset of cardiovascular disease as a predictor for offspring age of onset of cardiovascular disease. PLoS ONE 11(12):e0163334
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American heart association. Circulation 105:539–542
Allahwala U, Kott K, Bland A, Ward M, Bhindi R (2019) Anatomical assessment of coronary cllaterals predicts procedural success in patients undergoing chronic total occlusion percutaneous coronary intervention (CTO-PCI). Heart Lung Circ 28(4):S382
Schumacher SP, Everaars H, Stuijfzand WJ, Huynh JW, van Diemen PA, Bom MJ, de Winter RW, van Loon RB, van de Ven PM, van Rossum AC, Opolski MP, Nap A, Knaapen P (2020) Coronary collaterals and myocardial viability in patients with chronic total occlusions. EuroIntervention 16:453–461
Hakimzadeh N, Piek JJ (2013) The coronary collateral circulation revisited. Neth Heart J. 21(3):144–145
van Royen N, Piek JJ, Schaper IBIHW (2001) Stimulation of arteriogenesis; a new concept for the treatment of arterial occlusive disease. Cardiovasc Res 49(3):543–553
Cai W, Schaper W (2008) Mechanisms of arteriogenesis. Acta Biochim Biophys Sin (Shanghai) 40(8):681–692
van Oostrom MC, van Oostrom O, Quax PHA, Verhaar MC, Hoefer IE (2008) Insights into mechanisms behind arteriogenesis: what does the future hold? J Leukoc Biol. https://doi.org/10.1189/jlb.0508281
Galassi A (2009) Galassi’s Tips and Tricks. In: Galassi A (ed) Percutaneous Coronary Interventions for Chronic Total Occlusions. Springer, NY, pp 1–331
Berry C, Balachandran KP, L'Allier PL, Lespérance J, Bonan R, Oldroyd KG (2007) Importance of collateral circulation in coronary heart disease. Eur Heart J 28(3):278–291
Baroldi G, Mantero O, Scomazzoni G (1956) The collaterals of the coronary arteries in normal and pathologic hearts. Circ Res 4(2):223–229
Wellnhofer E, Olariu A, Klein C, Gräfe M, Wahl A, Fleck E, Nagel E (2004) Magnetic resonance low-dose dobutamine test is superior to SCAR quantification for the prediction of functional recovery. Circulation 109(18):2172–2174
Leone A, Landini L (2013) Vascular pathology from smoking: look at the microcirculation! CurrVasc Pharmacol 11(4):524–530
Maas AHEM, Appelman YEA (2010) Gender differences in coronary heart disease. Neth Heart J 18(12):598–602
Tiefenbacher CP, Friedrich S, Bleeke T, Vahl C, Chen X, Niroomand F (2004) ACE inhibitors and statins acutely improve endothelial dysfunction of human coronary arterioles. Am J Physiol Heart Circ Physio. https://doi.org/10.1152/ajpheart.00783.2003
Santra S, Basu AK, Roychowdhury P, Banerjee R, Singhania P, Singh S, Datta UP (2011) Comparison of left ventricular mass in normotensive type 2 diabetes mellitus patients with that in the nondiabetic population. J Cardiovasc Dis Res 2(1):50–56
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pirnat, M., Stillman, A.E., Rienmueller, R. et al. Can the degree of coronary collateralization be used in clinical routine as a valid angiographic parameter of viability?. Int J Cardiovasc Imaging 37, 379–388 (2021). https://doi.org/10.1007/s10554-020-01984-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-01984-5