Abstract
To assess left ventricular myocardial native T1/T2 values and systolic strain and their associations with B-type natriuretic peptide (BNP) and dialysis vintage in hemodialysis (HD) patients with a preserved left ventricular ejection fraction (LVEF). Forty-three HD patients with end-stage renal disease (ESRD) but a preserved LVEF (≥ 50%) and 28 healthy volunteers were enrolled. BNP was measured at the time of cardiac magnetic resonance (CMR) measurements. Global native T1 and T2 values were significantly higher in the HD patients (native T1: 1056 ± 32 ms vs. 1006 ± 25 ms, p < 0.001; T2: 50 ± 3 ms vs. 46 ± 2 ms, p < 0.001) than in the controls. The mean peak global circumferential strain (GCS) and global longitudinal strain (GLS) were both significantly reduced in the HD patients compared with the controls (GCS: − 13 ± 3 vs. − 16 ± 3, p < 0.001; GLS: − 12 ± 4 vs. − 15 ± 3, p = 0.001). In the HD patients, the global native T1 value showed a positive correlation with the global T2 value (r = 0.311, p = 0.042) and significant correlations with GCS (r = 0.564, p < 0.001) and GLS (r = 0.359, p = 0.018). Significant positive correlations were found between lg BNP levels and T2 values (r = 0.569, p < 0.0001) and the left atrial volume index (LAVI) (r = 0.536, p = 0.012). GLS showed significant positive correlations with the LVMI (r = 0.354, p = 0.020) and dialysis vintage (p = 0.026; r = − 0.339) in the HD patients. HD patients with a preserved LVEF have increased native T1/T2 values and decreased strain compared to controls. T2 values and the LVAI were positively associated with BNP in HD patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) and end-stage renal disease (ESRD) have high mortality rates despite significant advances in hemodialysis (HD), and cardiovascular disease (CVD) is a major cause of morbidity and mortality in HD patients [1,2,3]. Left ventricular hypertrophy (LVH) is a very common cardiac finding in ESRD patients [4]. Additionally, postmortem and biopsy studies have demonstrated that patients with ESRD have high levels of fibrosis, which is associated with an increased risk of sudden cardiac death [2, 5]. Previous studies revealed that this pattern of fibrosis is greater in HD patients than in patients with milder CKD and is progressively more severe with increasing dialysis vintage [1, 3]. However, other studies [6, 7] showed that HD patients have better LV systolic function than CKD patients before HD. The cardiovascular morphology and function in long-term HD patients are not well described. Since the LV ejection fraction (EF) is often preserved (EF ≥ 50%) in most HD patients with LVH [8], early detection and treatment of myocardial abnormalities and dysfunction is essential for the prevention and management of cardiomyopathy in HD patients.
The use of cardiac magnetic resonance imaging (CMRI) T1 mapping to quantify diffuse myocardial fibrosis has been validated in many previous histological studies [9,10,11]. Some recent studies revealed that HD patients had increased native myocardial T1 values and decreased strain compared with healthy subjects [12, 13], but they cannot exclude the possibility of an effect of myocardial edema from fluid shifts on native T1 values [12]. Evaluating T2 values is a good technique for detecting myocardial edema [14, 15].
In addition, the level of B-type natriuretic peptide (BNP), which is synthesized in the ventricular myocardium in response to ventricular diastolic and wall stress, is an independent predictor of cardiovascular death and overall mortality in patients undergoing dialysis [16]. The BNP level was reported to be increased in HD patients [17] and in patients with CKD not yet requiring dialysis therapy [18]. However, little is known regarding the associations between BNP and CMRI-derived cardiac indices in stable HD patients.
Consequently, we aimed to assess LV myocardial native T1/T2 values and systolic strain and their associations with traditional markers of increased cardiac risk, namely, BNP and dialysis vintage, in HD patients with a preserved left ventricular ejection fraction (LVEF).
Materials and methods
Study population
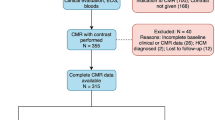
We prospectively recruited 43 HD patients with a preserved LVEF (≥ 50%) and 28 healthy volunteers of similar age, sex and body mass index (BMI) from the nephrology department of Wuhan Union Hospital and the community, respectively.
The inclusion criteria for HD patients were maintenance hemodialysis for 4-h sessions three times a week for ≥ 3 months; clinically confirmed CKD; age between 30 and 80 years; no clinical manifestations of chest pain, dyspnea, and palpitations; no history of heart disease (congenital heart disease, coronary artery disease, valvular heart disease or cardiomyopathy); and normal electrocardiographic manifestations. The inclusion criteria for the controls were as follows: age from 30 to 80 years; no history of heart disease, hypertension, diabetes or hyperlipidemia; normal physical examination; and normal electrocardiographic manifestations. The exclusion criteria for HD patients were a history of peritoneal dialysis or renal transplant with an abnormal LVEF (< 50%) by CMR; an inability to undergo MRI scanning (due to metal implants, severe claustrophobia); poorly controlled hypertension, blood glucose or blood lipids; and inadequate CMR image quality. Data regarding demographics, medical comorbidities, dialysis vintage, hematology, and serum biochemistry were collected prospectively (Table 1). The HD patients were divided into three groups according to dialysis vintage: group A (dialysis vintage ≤ 3 years; n = 21), group B (dialysis vintage > 3 years and ≤ 5 years; n = 11) and group C (dialysis vintage > 5 years; n = 10). This study was approved by the ethics committee of the Tongji Medical College of Huazhong University of Science and Technology. The present study was conducted in accordance with the Helsinki Declaration. We confirm that all methods in the study were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all participants.
HD patient biomarkers and other clinical parameters
Data for parameters tested in blood collected at the time of imaging were obtained from electronic records, including hemoglobin, albumin, C-reactive protein, phosphate, parathyroid hormone (PTH), glucose, and predialysis creatinine and potassium levels; the urea reduction ratio; lipid profiles; BNP levels; and each HD participant’s medical and dialysis history. Blood pressure was measured in the patients before an HD session. Patients were defined as hypertensive when the average systolic blood pressure (SBP) was greater than 140 mmHg. BMI was calculated by dividing dry weight (kg) by body height (m)2. The plasma BNP level was determined by radioimmunoassay using a Triage immunofluorescence diagnostic instrument produced by Biosite, USA.
CMRI scanning protocol
Patients and controls were scanned with a 1.5-T MRI scanner (MAGNETOM Area, Siemens Healthcare, Erlangen, Germany) with vector electrocardiogram gating and 18-channel phased array surface coils. Dialysis patients were all scanned on nondialysis days but not after a long break; thus, all scans were conducted between 18 and 24 h after the most recent dialysis session [12]. Cells of the LV long axis and short axis (coverage from the base to the top region) were obtained by a balanced steady-state free parade (b-SSFP) sequence. The cine acquisition parameters were as follows: repetition time, 2.93 ms; echo time, 1.16 ms; slice thickness, 6 mm; flip angle, 80°; field of view, 340 × 255 mm; and matrix, 256 × 205.
The prototype modified look-locker inversion recovery (MOLLI) sequence was used to generate native T1 maps at the base, middle and top levels of the LV short axis. The T1 mapping acquisition parameters were as follows: repetition time, 3.89 ms; echo time, 1.12 ms; slice thickness, 8 mm; flip angle, 35°; field of view, 360 × 270 mm; and matrix, 256 × 192. The T2 values of the LV myocardium were measured on a T2 map generated using a single-shot SSFP technique prepared by T2. The T2 mapping acquisition parameters were as follows: repetition time, 3.244 ms; echo time, 1.35 ms; slice thickness, 8 mm; flip angle, 70°; field of view, 360 × 75 mm; and matrix, 192 × 83.
Assessment of cardiac volume and function
A commercial postprocessing software program (Argus, Siemens Healthineers) was used offline to analyze cardiac structure and function. Cardiac volumetric and functional parameters were quantified based on manual delineation of the endocardial and epicardial borders using a stack of continuous short-axis slice cine images (after excluding papillary muscles from the myocardium). The LV end diastolic volume (EDV), end systolic volume (ESV), EF, stroke volume (SV), Cardiac Index (CI) and myocardial mass index (LVMI) were obtained automatically. In addition, left atrial volumes (LAVs) were calculated according to the biplane area-length method (LAV = [0.85 × (2-chamber area) × (4-chamber area)]/L, where L is the shortest dimension between the above two chambers) [19]. All of the above measurements were indexed to body surface area (BSA).
Native T1 and T2 mapping measurement
Native T1 and T2 values were measured by manually delineating regions of interest in the mid-layer of the myocardium of the basal, middle and apical LV segments. The 16 regions of interest in each volunteer were drawn based on the American Heart Association 16-segment model [20] (Fig. 1). The susceptibility to motion artifacts of each individual part was evaluated. Any segments with artifacts affecting the measurements were eliminated. After any segment was removed, the global T1/T2 time was calculated from the average to calculate all the remaining values. To determine the reproducibility of myocardial strain measurements, the same images of 20 randomly selected individuals were repeatedly measured by the same observer and another blinded observer independently.
Examples of end-diastolic cine images and corresponding T1 and T2 parametric maps in 1 hemodialysis (HD) patient and 1 control. The parts labeled 1a show the left ventricular middle short-axis segment, and the parts labeled 1b are the native T1 parametric maps at the same slice position in the same patient. The parts labeled 1c are the T2 parametric maps at the same slice position in the same patient. The parts labeled with 1 correspond to a 64-year-old HD patient. The mean global T1 value is 1139 ms, and the mean global T2 value is 54 ms. The parts labeled 2a show the left ventricular middle short-axis segment, and the parts labeled 2b are the native T1 parametric maps at the same slice position in the same person. The parts labeled 2c are the T2 parametric maps at the same slice position in the same person. The parts labeled with 2 correspond to a 65-year-old healthy volunteer. The mean global T1 value is 998 ms, and the mean global T2 value is 45 ms
Myocardial systolic strain measurement
Three-dimensional tissue tracking was performed offline using dedicated commercial software (TrufiStrain, version 2.0; Siemens Healthcare, Erlangen, Germany) based on heart deformation analysis (HDA). The short-axis cine images and two long-axis cine images were imported into this software. The LV endocardial and epicardial contours at the end of diastole were manually delineated on the short-axis and two long-axis cine images. The trabeculae and papillary muscles were included in the LV cavity. The LV global longitudinal strain (GLS), global circumferential strain (GCS), global radial strain (GRS) and early systolic strain rate were calculated by automatically tracking contours in each cardiac cycle (Fig. 2). To determine the reproducibility of the myocardial strain measurements, the same images from 15 randomly selected individuals were repeatedly measured by the same blinded observers.
Diagram of the peak systolic strain analysis of the left ventricular myocardium in a healthy volunteer by HDA software. The colored tissue-tracking maps from radial (a), circumferential (b), and longitudinal (c) strain analyses are shown on the left. The longitudinal (d), circumferential (e), and radial (f) strain values in a 16-segment model are displayed in the middle. The longitudinal (g), circumferential (h), and radial (i) strain–time curves in a cardiac cycle are shown on the right
Repeatability analysis
To determine the reproducibility of myocardial native T1 and T2 value and strain measurements, 15 individuals were randomly selected for repeatability analysis. The analysis was performed independently by two experienced radiologists, with one observer measuring the values again 2 weeks later. The measured data were used for consistency evaluations within and between observers. The mean values between the observers were taken as the results.
Statistical analysis
Statistical analyses of all data were carried out using SPSS software (SPSS 21.0 for Windows, IBM, Chicago, IL, USA). For all continuous data, the normality of the distribution was assessed using the Kolmogorov–Smirnov test. Normally and nonnormally distributed data and categorical variables are expressed as the means ± standard deviations, medians (interquartile ranges) and frequencies (percentages), respectively. The independent-sample Student’s t test was used to compare two groups of normally distributed variables, and the chi-square test was used to compare categorical variables. Normally distributed variables were analyzed by Pearson’s correlation, and nonnormally distributed data were analyzed by the Spearman correlation (log-transformed BNP levels and PTH). Multiple linear regression analyses were performed to identify determinants of myocardial native T1/T2 values and GLS in patients with HD. All candidate variables (p < 0.10 in univariable linear regression and without collinearity) were entered into a multiple stepwise regression model. Clinical characteristics and CMRI findings were compared among groups A, B and C by one-way ANOVA. A p value < 0.05 (two-tailed) was considered statistically significant.
Results
Clinical characteristics of the study population
A total of 71 subjects were enrolled: 43 HD patients and 28 healthy volunteers. The baseline demographic characteristics of the HD patients and the prescribed medications used in this group are shown in Table 1. The controls had no cardiovascular or systemic diseases and a normal electrocardiogram. The controls were not treated for hypertension or hypercholesterolemia and were not taking regular medications.
Mass, volume and function of the left ventricle
Compared with those in the controls, the LAVI, LVMI, EDV and ESV were significantly increased (LAVI: 42 ± 21 vs. 36 ± 13, p = 0.009; LVMI: 100 ± 33 vs. 58 ± 10 g/m2, p < 0.001; EDV: 76 ± 25 vs. 54 ± 10 mL/m2, p < 0.001; ESV: 33 ± 20 vs. 20 ± 7 mL/m2, p < 0.001) in the HD patients. However, no significant difference in the LVEF was found between the two groups (61 ± 8 vs. 68 ± 10%, p = 0.766). The lg BNP levels were significantly associated with the LAVI (r = 0.536, p = 0.012).
Native T1 and T2 values and strain
The global native T1 and T2 values were significantly higher in the HD patients than in the controls (native T1: 1056 ± 32 ms vs. 1006 ± 25 ms, p < 0.001; T2: 50 ± 3 ms vs. 46 ± 2 ms, p < 0.001) (Table 2) (Fig. 3a, b). Furthermore, we found that the native T1 values and T2 values of 16 segments of the left ventricle in the HD patients were higher than those in the healthy controls (Fig. 4a, b). The native T1 and T2 values showed no significant differences between the septal and nonseptal regions in the HD patients or in the controls (native T1: 1066 ± 38 ms vs. 1052 ± 31 ms, p = 0.062; T2: 50 ± 3 ms vs. 50 ± 3 ms, p = 0.269) (Table 2). The mean peak GCS and GLS were both significantly reduced in the HD patients compared with the controls (GCS: − 13 ± 3 vs. − 16 ± 3, p < 0.001; GLC: − 12 ± 4 vs. − 15 ± 3, p = 0.001) (Fig. 3c, d). No difference in GRS was found between the HD and control groups (39 ± 12 vs. 43 ± 10, p = 0.126) (Table 2).
After adjustment for heart rate (HR), the native T1 and T2 values and peak GCS and GLS were still significantly different between the two groups (Tables 3, 4).
Factors associated with myocardial native T1 and T2 values
The global native T1 value was correlated with gender (r = 0.328, p = 0.032). The global native T1 value showed a positive correlation with the global T2 value (r = 0.311, p = 0.042) (Fig. 5a) as well as significant correlations with GCS (r = 0.564, p < 0.001) and GLS (r = 0.359, p = 0.018) in the HD patients but not in the control subjects. Significant positive correlations were found between the T2 value and GCS (r = 0.346, p = 0.023) and lg BNP(log-transformed) levels (r = 0.569, p < 0.0001) (Fig. 5b). On multivariable analysis, the independent determinants of the native T1 value were gender (β = 0.365, p = 0.007) and lg BNP levels (β = 0.365, p = 0.007) (model R2 = 0.271) (Table 6). The independent determinant of the T2 value was the lg BNP level (β = 0.545, p < 0.001) (model R2 = 0.280).
a The relationship between native myocardial T1 and T2 values in hemodialysis patients; b the relationship between peak global longitudinal strain and the left ventricular mass index; c the relationship between native myocardial T2 values and lg BNP level in hemodialysis patients; d comparison of peak global longitudinal strain among the groups of different dialysis vintages (group A: dialysis vintage ≤ 3 years; group B: dialysis vintage > 3 years and ≤ 5 years; and group C: dialysis vintage > 5 years)
Factors associated with myocardial strain
GLS was found to be positively correlated with LVEF and the LVMI and negatively correlated with dialysis vintage in HD patients (LVEF: r = − 0.344, p = 0.024; LVMI: r = 0.354, p = 0.026; r = − 0.339, p = 0.020) (Fig. 5c, d). GRS showed a significant positive correlation with LVEF in the HD patients (GRS: r = 0.548, p < 0.001). GCS showed a significant negative correlation with LVEF in the HD patients (GCS: r = − 0.385, p = 0.011) (Table 5). On multivariable analysis, the independent determinant of GLS was dialysis vintage (β = − 0.339, p = 0.029) (model R2 = 0.094) (Table 6).
In addition, GLS was independently correlated with dialysis vintage (standardized r = − 0.321, p = 0.044). GLS was increased in the HD patients in group C compared with that in group A (p = 0.008) and group B (p = 0.011) patients (Fig. 5d). No significant differences in sex, BMI, age, EF, EDV, ESV, LVMI or native T1/T2 values were identified between the three groups (Table 7).
Intra- and interobserver reproducibility
The intraclass correlation coefficients (ICCs) in the intraobserver analysis were 0.977, 0.978, 0.980, 0.967, and 0.923 for the native T1 and T2 values, GRS, GCS, and GLS, respectively. The ICCs in the interobserver analysis were 0.967, 0.969, 0.971, 0.956 and 0.912 for the native T1 and T2 values, GRS, GCS, and GLS, respectively.
Discussion
ESRD patients with a preserved LVEF have been shown to have increased myocardial fibrosis [2, 5]. In this study, native T1 values were significantly higher and were associated with decreased GCS and GLS in the HD patients compared with the healthy controls, which is consistent with previous studies [12, 13]. Increased T1 values have been shown to be related to interstitial fibrosis in many previous histological studies [9,10,11]. However, native T1 values have been shown to be increased in several other diseases, including myocardial edema [21], acute myocardial infarction, amyloidosis [22], and nonischemic cardiomyopathy [23]. Because no patients in our HD group had a history of amyloidosis, no differences in the in rates of previous myocardial infarction were found between the HD and control groups. Thus, these conditions would be unlikely to interfere with our results. However, we cannot exclude the possibility of fluid shifts in myocardial edema affecting the native T1 values. Moreover, we found that the global T2 value was significantly higher in the HD patients than in the controls, although Graham et al. [24] showed that the reproducibility of native T1 mapping was excellent and unrelated to changes in markers of fluid status in HD patients. We found a positive weak but significant correlation between T2 values and native T1 values. Furthermore, Marlies et al. [25] demonstrated that native T1 values were significantly associated with fluid status, suggesting that chronic fluid overload may also modulate increased T1 values independent of fibrosis. Therefore, the high native T1 and T2 values in our study suggested that not only fibrosis but also interstitial edema or inflammation may potentially have a nonnegligible influence on HD patients. One possible mechanism is chronic inflammation of the heart in HD patients, which results in an increase in cardiomyocyte water exudation [26]. In addition, a significant positive correlation was found between the T2 value and GCS, indicating that myocardial edema was associated with decreased wall compliance.
BNP levels were reported to be increased in HD patients [17] and in patients with CKD not yet requiring dialysis therapy [18]. Moreover, previous studies found positive correlations between BNP levels and the LVMI and LVEF in HD patients [27, 28], suggesting that BNP levels are a good predictor of LV dysfunction. In the present study, positive correlations were found between BNP levels and T2 values and the LVAI in HD patients. High T2 values reflect myocardial edema or inflammation [14, 15], and the LAVI was proven to be markedly increased in ESRD patients and independently associated with LVH and LV systolic and diastolic dysfunction. Therefore, BNP may be a useful biomarker for detecting myocardial abnormalities and LA remodeling in HD patients.
To date, the effect of HD therapy on cardiac function in ESRD patients remains controversial. In our study, dialysis vintage was found to be independently correlated with GLS. Furthermore, we found that the HD patients in group C had markedly increased GLS values compared with those in the other two groups. One previous study showed that the process of progressive LVH continues after initiation of dialysis therapy, especially in the first year [3]. In a large-sample study, Kamyar et al. [29] demonstrated that greater fluid retention between two subsequent hemodialysis treatment sessions is associated with a higher risk of all-cause and cardiovascular death in HD patients. However, overhydration and accumulation of uremic toxins may influence the development of LVH and LV dysfunction in patients with chronic renal failure, suggesting that HD treatment may improve cardiac function by uremic toxin removal. Hayashi et al. [30] reported that myocardial function improves after one session of HD. Meanwhile, Liu et al. [6] showed that HD patients have better LV systolic function than moderate-advanced CKD patients by STE, and the better LV systolic function in HD patients may be due to the removal of uremic toxins [6]. Subsequently, Elaine et al. [7] also found that both GLS and the strain rate increased in patients following 6 months of HD. This result may also be due to an improvement in LV function or improved control of cardiovascular risk factors in the long dialysis vintage groups. Nevertheless, without a correlative analysis of the tissue, we cannot be certain that changes in GLS reflect an improvement in myocardial tissue abnormalities. Moreover, a possible explanation for more favorable findings in long-term survivors than in short-term HD patients is survival bias as patients with more severe cardiovascular characteristics died sooner while on dialysis [31]. Further studies are required to investigate the long-term effect of stable HD therapy on the cardiac function of patients, as well as the possible mechanisms.
Our study has some limitations. First, this was a single-center study with a relatively small cohort, and larger studies are required to confirm our findings and identify any potential prognostic benefits. Second, the lack of a histological correlation in this study is a limitation as this analysis could have provided further information. However, this limitation reflects the decreasing use of endomyocardial biopsy in routine clinical practice. Additionally, biopsy is limited by sampling error. Third, diastolic dysfunction and echocardiographic parameters were not available for our enrolled subjects. Finally, although our subjects included some HD patients with hypertension, which affects myocardial structure and function, we excluded patients with poorly controlled hypertension. Associations between blood pressure and CMR-derived cardiac indices were also not observed because of our exclusion of patients with poorly controlled hypertension according to the exclusion criteria. Howevver, a multivariable analysis was performed to exclude this confounding factor.
Conclusion
In conclusion, HD patients with a preserved LVEF have increased native T1/T2 values and decreased strain compared to controls. T2 values and the LVAI were positively associated with BNP in HD patients. Native T1/T2 mapping and strain may have the potential to quantify the severity of early cardiomyopathy in HD patients and monitor the progress of myocardial abnormalities with HD therapy. Future research with larger sample sizes should focus on investigating the long-term effect of stable HD therapy on the cardiac function of patients, as well as the possible mechanisms.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Mall G, Huther W, Schneider J, Lundin P, Ritz E (1990) Diffuse intermyocardiocytic fibrosis in uraemic patients. Nephrol Dial Transplant 5(1):39–44. https://doi.org/10.1093/ndt/5.1.39
Losi MA, Memoli B, Contaldi C, Barbati G, Del Prete M, Betocchi S, Cavallaro M, Carpinella G, Fundaliotis A, Parrella LS, Parisi V, Guida B, Chiariello M (2010) Myocardial fibrosis and diastolic dysfunction in patients on chronic haemodialysis. Nephrol Dial Transplant 25(6):1950–1954. https://doi.org/10.1093/ndt/gfp747
Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE (1998) Long-term evolution of cardiomyopathy in dialysis patients. Kidney Int 54(5):1720–1725. https://doi.org/10.1046/j.1523-1755.1998.00154.x
Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE (1995) Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 47(1):186–192. https://doi.org/10.1038/ki.1995.22
Edwards NC, Moody WE, Chue CD, Ferro CJ, Townend JN, Steeds RP (2014) Defining the natural history of uremic cardiomyopathy in chronic kidney disease: the role of cardiovascular magnetic resonance. JACC Cardiovasc Imaging 7(7):703–714. https://doi.org/10.1016/j.jcmg.2013.09.025
Liu YW, Su CT, Huang YY, Yang CS, Huang JW, Yang MT, Chen JH, Tsai WC (2010) Left ventricular systolic strain in chronic kidney disease and hemodialysis patients. Am J Nephrol 33(1):84–90. https://doi.org/10.1159/000322709
Rutherford E, Mangion K, McComb C, Bell E, Cockburn S, Talle M, Roditi G, Welsh P, Woodward R, Radjenovic A, Struthers AD, Jardine AG, Berry C, Patel RK, Mark PB (2017) Myocardial changes in incident haemodialysis patients over 6-months: an observational cardiac magnetic resonance imaging study. Sci Rep 7(1):13976. https://doi.org/10.1038/s41598-017-14481-y
Liu YW, Su CT, Sung JM, Wang SP, Su YR, Yang CS, Tsai LM, Chen JH, Tsai WC (2013) Association of left ventricular longitudinal strain with mortality among stable hemodialysis patients with preserved left ventricular ejection fraction. Clin J Am Soc Nephrol 8(9):1564–1574. https://doi.org/10.2215/CJN.10671012
Ide S, Riesenkampff E, Chiasson DA, Dipchand AI, Kantor PF, Chaturvedi RR, Yoo S-J, Grosse-Wortmann L (2017) Histological validation of cardiovascular magnetic resonance T1 mapping markers of myocardial fibrosis in paediatric heart transplant recipients. J Cardiovasc Magn Reson 19(1):10. https://doi.org/10.1186/s12968-017-0326-x
Mewton N, Liu CY, Croisille P, Bluemke D, Lima JAC (2011) Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 57(8):891–903. https://doi.org/10.1016/j.jacc.2010.11.013
Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, Francis JM, Karamitsos TD, Prendergast BD, Robson MD (2013) Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 99(13):932–937. https://doi.org/10.1136/heartjnl-2012-303052
Graham-Brown MP, March DS, Churchward DR, Stensel DJ, Singh A, Arnold R, Burton JO, McCann GP (2016) Novel cardiac nuclear magnetic resonance method for noninvasive assessment of myocardial fibrosis in hemodialysis patients. Kidney Int 90(4):835–844. https://doi.org/10.1016/j.kint.2016.07.014
Rutherford E, Talle MA, Mangion K, Bell E, Rauhalammi SM, Roditi G, McComb C, Radjenovic A, Welsh P, Woodward R, Struthers AD, Jardine AG, Patel RK, Berry C, Mark PB (2016) Defining myocardial tissue abnormalities in end-stage renal failure with cardiac magnetic resonance imaging using native T1 mapping. Kidney Int 90(4):845–852. https://doi.org/10.1016/j.kint.2016.06.014
Montant P, Sigovan M, Revel D, Douek P (2015) MR imaging assessment of myocardial edema with T2 mapping. Diagn Interv Imaging 96(9):885–890. https://doi.org/10.1016/j.diii.2014.07.008
Giri S, Chung Y-C, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP (2009) T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson 11(1):56. https://doi.org/10.1186/1532-429X-11-56
Carr SJ, Sunita B, Barbara F, Leong N (2005) Prognostic potential of brain natriuretic peptide (BNP) in predialysis chronic kidney disease patients. Clin Sci 109(1):75–82. https://doi.org/10.1042/CS20040351
Roberts MA, Hare DL, Ratnaike S, Ierino FL (2006) Cardiovascular biomarkers in CKD: pathophysiology and implications for clinical management of cardiac disease. Am J Kidney Dis 48(3):341–360. https://doi.org/10.1053/j.ajkd.2006.06.005
Yoichi T, Takeshi H, Yoshio I, Shin T, Kei K, Fumiki Y, Satoko N, Hajime N, Takashi I, Kenji K (2004) Diagnostic and prognostic value of plasma brain natriuretic peptide in non-dialysis-dependent CRF. Am J Kidney Dis 44(3):420–428. https://doi.org/10.1053/j.ajkd.2004.05.023
Caudron J, Fares J, Bauer F, Dacher JN (2011) Evaluation of left ventricular diastolic function with cardiac MR imaging. Radiographics 31(1):239–259. https://doi.org/10.1148/rg.311105049
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennel DJ, Rumberger JA, Ryan T, Verani MS (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105:539–542. https://doi.org/10.1161/hc0402.102975
Ferreira VM, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Choudhury RP, Friedrich MG, Robson MD, Neubauer S (2012) Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson 14(1):42. https://doi.org/10.1186/1532-429X-14-42
Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, Whelan CJ, Myerson SG, Robson MD, Hawkins PN, Neubauer S, Moon JC (2013) Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging 6(4):488–497. https://doi.org/10.1016/j.jcmg.2012.11.013
Shah RV, Kato S, Roujol S, Murthy V, Bellm S, Kashem A, Basha T, Jang J, Eisman AS, Manning WJ, Nezafat R (2016) Native myocardial T1 as a biomarker of cardiac structure in non-ischemic cardiomyopathy. Am J Cardiol 117(2):282–288. https://doi.org/10.1016/j.amjcard.2015.10.046
Graham-Brown MP, Rutherford E, Levelt E, March DS, Churchward DR, Stensel DJ, Mccomb C, Mangion K, Cockburn S, Berry C (2017) Native T1 mapping: inter-study, inter-observer and inter-center reproducibility in hemodialysis patients. J Cardiovasc Magn Reson 19(1):21. https://doi.org/10.1186/s12968-017-0337-7
Antlanger M, Aschauer S, Kammerlander AA, Duca F, Saemann MD, Bonderman D, Mascherbauer J (2018) Impact of systemic volume status on cardiac magnetic resonance T1 mapping. Sci Rep 8(1):5572. https://doi.org/10.1038/s41598-018-23868-4
Ronco C, Haapio M, House AA, Anavekar N, Bellomo R (2008) Cardiorenal syndrome. J Am Coll Cardiol 52(19):1527–1539. https://doi.org/10.1016/j.jacc.2008.07.051
Nishikimi T, Futoo Y, Tamano K, Takahashi M, Suzuki T, Minami J, Honda T, Uetake S, Asakawa H, Kobayashi N (2001) Plasma brain natriuretic peptide levels in chronic hemodialysis patients: influence of coronary artery disease. Am J Kidney Dis 37(6):1201–1208. https://doi.org/10.1053/ajkd.2001.24523
Naganuma T, Sugimura K, Wada S, Yasumoto R, Sugimura T, Masuda C, Uchida J, Nakatani T (2002) PayPerView: the prognostic role of brain natriuretic peptides in hemodialysis Patients—Karger publishers. Am J Nephrol. https://doi.org/10.1159/000065272
Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, Fonarow GC (2009) Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 119(5):671–679. https://doi.org/10.1161/CIRCULATIONAHA.108.807362
Hayashi SY, Brodin LA, Alvestrand A, Lind B, Stenvinkel P, do Nascimento M, Qureshi AR, Saha S, Lindholm B, Seeberger A (2004) Improvement of cardiac function after haemodialysis. Quantitative evaluation by colour tissue velocity imaging. Nephrol Dial Transplant 19(6):1497–1506. https://doi.org/10.1093/ndt/gfh205
Thompson RB, Raggi P, Wiebe N, Ugander M, Nickander J, Klarenbach SW, Thompson S, Tonelli M, Alberta Kidney Disease N (2019) A cardiac magnetic resonance imaging study of long-term and incident hemodialysis patients. J Nephrol. https://doi.org/10.1007/s40620-019-00593-3
Acknowledgements
We would like to thank all our colleagues for helping us during the current study. We are also very grateful to all the selfless volunteers who participated in the study.
Funding
No funding was received for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
This study was approved by the ethics committee of Tongji Medical College of Huazhong University of Science and Technology.
Informed consent
All subjects provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, X., He, F., Cao, Y. et al. Associations of B-type natriuretic peptide (BNP) and dialysis vintage with CMRI-derived cardiac indices in stable hemodialysis patients with a preserved left ventricular ejection fraction. Int J Cardiovasc Imaging 36, 2265–2278 (2020). https://doi.org/10.1007/s10554-020-01942-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-01942-1