Abstract
Cardiac adaptations to exercise on an elite level have been well studied. Strain analysis by speckle tracking echocardiography has emerged as a tool for sports cardiologists to assess the nature of hypertrophy in athletes’ hearts. In prior studies, strain values generally did not change in physiological adaptations to exercise but were reduced in pathological hypertrophy. However, research in this field has focused almost solely on male athletes. Purpose of the present study is to investigate strain values in the hearts of female elite athletes in football and volleyball. In this cross-sectional study echocardiography was performed on 19 female elite football-players, 16 female elite volleyball-players and 16 physically inactive controls. Conventional echocardiographic data was documented as well as left ventricular longitudinal, radial and circumferential strain values gained by speckle tracking echocardiography. The hearts of the female athletes had a thicker septal wall, a larger overall mass and larger atria than the hearts in the control group. Global longitudinal, radial and circumferential strain values did not differ between the athletes and controls or between sporting disciplines. No correlation between septal wall thickness and global strain values could be documented. Cardiac adaptations to elite level exercise in female volleyball and football players do not influence global strain values. This has been documented for male athletes of several disciplines. The present study adds to the very limited control-group comparisons of left ventricular strain values in elite female athletes. The findings indicate that global strain values can be used when assessing the cardiac health in female athletes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Structural and functional cardiac adaptations to physical activity as performed by elite athletes are a well-studied phenomenon. Especially athlete’s heart (AH), a myocardial hypertrophy as a reaction to a high endurance training load has been the focus of intense research [1]. However, the overwhelming majority of evidence regarding AH has been derived from studies focusing on male athletes [2]. A trend that has continued regarding the relatively novel echocardiographic technique of strain analysis (SA) by speckle tracking echocardiography (STE). This technique has added a new element to the chronic struggle of the sports cardiologist in differentiating between physiological adaptations to exercise and pathological entities with an increased potential for sudden cardiac death such as hypertrophic cardiomyopathy [3]. In prior studies, global strain values generally did not change in physiological adaptations to exercise but were reduced in pathological hypertrophy [4,5,6].
Speckle tracking echocardiography (STE) has gained growing attention in the last decade. The automatic tracking of groups of speckles allows for an objective quantification of cardiac function in several dimensions. Longitudinal strain is derived from apical images, while circumferential and radial strain is measured in the parasternal short axis. Longitudinal strain describes a kinetic action in percent of the myocardium decreasing the basal–apical distance. Circumferential strain describes myocardial shortening in percent in a circular manner in the short axis, while radial strain depicts the thickening of the myocardium during contraction in the short axis. Longitudinal and circumferential strain values are stated as negative percent to portray a “shortening”, while radial strain is stated as a positive value [5, 7]. Inter- and intraobserver variability of left ventricular (LV) global longitudinal strain (GLS) is comparable to the classic planimetric measurement of ejection fraction, while variability is somewhat higher for radial and circumferential measurements [7].
The effects of exercise on a recreational and competitive level on myocardial strain patterns have been the focus of several studies. Even though results vary between studies, large reviews and meta-analyses reach the conclusion that LV-GLS should not normally be reduced in athletes compared to controls, even when physiological hypertrophy is present [5, 8]. Regarding global circumferential strain (GCS) and global radial strain (GRS) studies are even fewer but may indicate differences between certain athletic populations and controls [8].
However, these insights are derived almost solely from data of male athletes. The few studies including female athletes usually analyze the whole athlete cohort without sub-analysis by gender, fail to specify sporting disciplines or include very few female athletes. The largest cohort of female athletes (n = 78/200) was included by Caselli et al., who found that the GLS was slightly higher in females (athletes and controls) compared to males [9]. Sporting disciplines were not specified exactly. In the same year D’Ascenzi et al. reported no difference in GLS when analyzing 36 female and 55 male athletes from different sporting disciplines [10]. D’Ascenzi et al. also published left ventricular strain data of 24 female volleyball athletes in a study focused on atrial and right ventricular strain [11]. To the authors’ knowledge to date less than 150 cases of 2D-STE derived left ventricular strain of female athletes have been published from controlled studies collectively. Also, regarding female athletes most athletic disciplines have been left untouched or not been specifically reported.
The goal of this study is to shed light on the cardiac structures of female elite football- and volleyball-players compared to non-athletic controls with a focus on strain analysis by speckle tracking echocardiography.
Methods
Hypothesis
Hypothesis I: The female athletes will have a larger diastolic ventricular septum diameter than the controls. Hypothesis II: The global longitudinal, radial and circumferential strain values will not differ between the groups and will not correlate with septal diameter.
Participants
We recruited 19 female football players (FP) from a Bundesliga top team, 16 volleyball players (VP) from a Bundesliga top team and 16 age-matched non-athletic female controls (C). All participants were caucasian, free of known cardiac disease or other major morbidity and healthy at the time of diagnostic testing. No exercise was performed acutely (> 10 h) before the echocardiography. The elite athletes had a minimum of 5 years competitive club level experience with at least 6 weekly hours of training for the last 3 years. The control group participants had never engaged in club level or competitive sport and exercised < 2 h per week for the last 5 years at least.
Procedure
An oral history and physical examination by an experienced sports physician and a questionnaire were conducted to assess health and exercise history. Weight and height were documented in all participants, as well as body composition in the volleyball players and controls (Seca, Germany, 2018), followed by echocardiography.
Echocardiography
Echocardiography was performed for all participants by the same experienced echocardiographer using a Vivid iq with a M5Sc 1.5–4.6 transducer (GE Healthcare Systems, USA, 2018), the participant in a semi-supine resting position. Images were stored based on the recommendations of the American Society of Echocardiography (ASE) while the participant held her breath.
Conventional echocardiography included standard measurements of cardiac dimensions, contractility and diastolic function (see Table 1 for details). Measurements were taken as advised by the American Society of Echocardiography (ASE) [12]. To calculate volumes and ejection fraction Simpson’s biplane-method was used. Diastolic parameters were derived by assessing LV-filling pressures using peak E and A velocities and the E/e′-ratio (e′ measured at medial mitral annulus) [13].
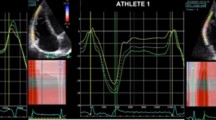
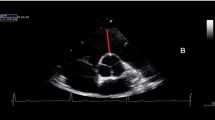
Speckle Tracking images for calculation of myocardial strain were recorded in apical 3-chamber, 2-chamber and 4-chamber views for longitudinal values (Image 1) and in the parasternal short-axis at the level of papillary muscles for circumferential and radial values (Images 2 and 3). The frame rate was set between 40 and 90 frames per second and an exact endocardial and epicardial delineation was attempted for all images. Analysis of all images was conducted offline by the same experienced echocardiographer using EchoPac-Software (Version 202, GE Healthcare, USA, 2018) while being blinded to the group membership of the participant during the analysis. The region of interest (ROI) was placed semi-automatically by the program and adjusted by the analyst to optimally encompass the whole myocardium. Global longitudinal strain (GLS) was calculated as the average of 18 segments (3 views à 6 segments) (Image 4), global circumferential strain (GCS) and global radial strain (GRS) were derived as the average of six separate myocardial segments from a parasternal view. Segments without clear myocardial delineation or inability of the software to adequately track speckles were excluded from further analysis, calculating averages based on the remaining segments.
Statistical analysis
All data was accumulated in Excel (Microsoft Corporation, Redmond, USA, 2019) and analyzed using SPSS Version 26 (IBM Corporation, Armonk, USA). Normal distribution was assessed using a Kolmogorov–Smirnoff test. Comparisons between groups of normally distributed parameters were analyzed using an independent T-Test. Correlations were analyzed using a Pearson test for normally distributed and a Spearman test for non-normally distributed parameters. A p-value < 0.05 was defined as statistically significant for all tests.
Results
All groups were matched regarding age (Table 2), while height and weight were higher in the volleyball group. In the sub-group analysis of body composition no differences were seen in total fat mass (absolute and relative), while visceral fat mass was lower in VP than C and fat free mass (absolute and relative) was higher (Table 3).
Echocardiography
No athletes or controls had to be excluded; visibility and myocardial delineation for strain analysis were adequate in all participants. Very few individual segments were excluded from calculating strain averages.
Conventional 2D echocardiography
Conventional echocardiographic measurements are summarized in Table 1, while selected parameters are shown in Fig. 1. The interventricular septum diameter in diastole (IVSd) was larger in both athlete-populations compared to controls (Image 5) but not different between sporting disciplines. Only five athletes (4 FP and 1 VP) and no controls had IVSd ≥ 1 cm. The left ventricular internal diameter in diastole (LVIDd) was larger in VP than in FP and C; no difference could be noted for FP vs C. The left ventricular posterior wall in diastole (LVPWd) was thicker in athletes than controls and in VP than FP (Table 1). Athletes had larger atria than controls, a larger right ventricular basal diameter in diastole (RVBDd), larger left ventricular end diastolic volume (LVVED) and a larger stroke volume (LVSV). No differences were observed between sporting disciplines when indexed for body surface area (BSA). Ejection fraction (EF) was without significant difference between all groups, as were parameters of diastolic function. The length of the right ventricle in diastole (RVDl) was greater in VP than in the other two groups.
Selected parameters of the conventional echocardiography are shown as mean ± standard deviation of competitive football athletes (F), volleyball athletes (V) and controls (C). End-diastolic volume in ml (EDV), stroke volume in ml (SV), ejection fraction in % (EF), left atrial end-systolic volume (LAESV index), diastolic interventricular septum in cm (IVSd). *Indicates a significant difference compared to both other groups
Left: 4-chamber view of a football-athlete showing the eccentric hypertrophy typical of athlete’s heart; diastolic septal diameter 1.1 cm, left ventricular diastolic diameter 5.1 cm. Right: 4-chamber view of a control group participant; diastolic septal diameter 0.6 cm, left ventricular diastolic diameter 4.4 cm
Speckle tracking echocardiography
Strain-data is summarized in Table 4 and Fig. 2. No significant differences were documented in global longitudinal strain (GLS), global circumferential strain (GCS) or global radial strain (GRS) between the athletes and controls or between sporting disciplines. Only five segments of the whole cohort had to be excluded from analysis due to reduced trackability of speckles by the software. A detailed analysis of the individual segments did not reveal any relevant differences in any strain type when comparing the different groups of participants. However, when pooling segmental values into the three categories basal, mid-ventricular and apical the volleyball players (VP) showed a significantly lower apical longitudinal strain compared to football players (FP) and controls (C) (Fig. 3). No correlation between septal wall thickness (IVSd) and any of the global strain parameters could be documented. A comparison of global longitudinal strain-values between participants with septal wall thickness ≥ 1 cm and < 1 cm yielded no significant differences (p = 0.81).
Limitations
The primary limitation of the present investigation is the small sample size. With the aim of the present study being the analysis of potential norm value deviations in the cardiac mechanics of elite athletes the findings would have been more representative given a larger sample size. A further limitation is the smaller body size of the control group in comparison to the volleyball athletes. While being a fine comparator for the football athletes, the difference in body size may well limit the comparability of cardiac mechanics with the volleyball athletes; the elongated heart architecture of the tall athletes may well influence some of the investigated cardiac parameters [14]. Furthermore, an exact documentation of training hours would have allowed for a more detailed analysis and comparison along the lines of training volume and cardiac mechanics. While a minimum and a maximum of physical training for the athletes and controls respectively was part of the in- and exclusion criteria (see "Methods"), no details regarding by how much the athletes surpassed the threshold were analyzed. However, the fact that all athletes of the respective disciplines were team-mates allows for the estimation of similar training volumes. Lastly, the all-caucasian sample limits the transfer of the results to the general population of athletes considering the well-documented differences in cardiac wall thickness between athletes of different ethnicity [6, 15].
Discussion
As expected, the athletes in this study demonstrated cardiac adaptations that have been described in many prior investigations as an adaptation to high levels of physical exercise [1]. And while this study lacks the longitudinal design to investigate the effect of the specific sporting discipline on these adaptations, the observed differences between the athletes and control group regarding conventional echocardiographic parameters can be safely assumed to be exercise-related. Thus, the hearts of the athletes had a larger mass (absolute and indexed to body surface area), a thicker septum, larger atrial volumes and a wider right ventricle than the hearts in the control group. The heart rate of the athletes was lower and in the sub-group analysis the volleyball-players had a higher fat-free mass and less visceral fat than the control-group. The hypothesis that the athletes would present a larger ventricular septal diameter than the control group could not be falsified.
The three left venrticular strain parameters GLS, GRS and GCS did not differ between the sporting disciplines or between athletes and controls (Table 4); thus, hypothesis II could not be falsified. Figure 4 demonstrates the difference in septal diameter between athletes and control group, as well as a lack of difference in the global longitudinal strain values. This is in accordance with prior studies focusing on strain values in female athletes. D’Ascenzi et al. published the LV global longitudinal strain values of 24 female volleyball athletes, measured with the same vendor and software as the present study at − 19.7 ± 1.8 after detraining for 3 months and at − 20.5 ± 2.1 after 4 months of intense training; no LV-GLS data was published for the control group as the study focused on the atria and the right ventricle [11]. Caselli et al. published LV-GLS data from a large athlete cohort (n = 200, including 78 female athletes), finding LV-GLS-values in athletes within the normal range, but with slightly lower values when compared to the control group; LV-GLS was higher in females than males across the whole cohort. Similar GLS-values as in the present study were presented for the athletes overall, albeit using hard- and software of a different vendor [9]. Cappelli et al. included 9 female endurance athletes in their comparison of 50 endurance athletes with hypertensive patients and healthy controls: LV-GLS did not differ between the athletes and the control group; results were not specified by gender. The GLS-values were comparable to the results of the present study [16]. A position statement by Pelliccia et al. and a review by D’Ascenzi et al. present similar LV-GLS-values as the present study and conclude that even high training volumes in elite athletes do not normally reduce LV-GLS-values [5, 6]. A meta-analysis published by Beaumont et al. in 2017 included only male athletes, but thoroughly analyzed all available data, concluding that GLS-values do not differ between athletes of varying disciplines and controls; LV-GLS-values were comparable to the data of the present study. The authors did, however, find a correlation between LV-GLS and left ventricular mass index, suggesting a small LV-GLS-reduction associated with increasing physiological exercise-associated hypertrophy, interpreted by the authors as indicative of an increased functional reserve in the highly trained heart [8]. Possibly due to the fact that hypertrophy is more pronounced in male athletes the present study did not find this association between hypertrophy and LV-GLS-reduction, even in detailed regional analysis: To probe in more detail for an influence of physiological hypertrophy on strain values the anteroseptal and septal (3-chamber-view and 4-chamber-view respectively) longitudinal strain values and the septal segments in the short axis strain-values were analyzed regarding possible correlations between strain values and septal thickness; no significant correlations were observed.
The detailed comparison of segmental strain values between the groups did not reveal differences between individual segments, supporting the credibility of the similarity between the global values. The only deviation from the overall similar values between the groups was a slightly lower apical longitudinal strain in the elite volleyball players compared to both elite football players and controls (Fig. 3). Whether this finding is connected to differences in cardiac architecture and resulting contraction patterns (the hearts of the volleyball players were significantly longer, as befits the greater average height) or is a coincidental aberration must be elucidated in further studies revealing detailed segmental strain findings. Stefani et al. were able to demonstrate a basal–apical gradient with an increase in mid-apical longitudinal strain in male football players after an acute 3-min physical exertion (compared to the resting state beforehand) [17]. In their meta-analysis on cardiac strain in athletes, Beaumont et al. describe a higher apical dynamic in athletes, demonstrated by a reduced apical circumferential strain at rest, which increases during exercise [8]. Cappelli et al. report an increase in apical circumferential strain in hypertensive patients compared to athletes and controls, suggesting a compensatory mechanism of beginning reduced contractile function. Based on these observations, the reduction in apical strain in the volleyball athletes may be a sign of an apical contractile reserve that may differ from the football- and control-cohort due to the elongated LV-structure. This is, however, conjecture that should be investigated by regional strain analyses after exertion in future studies in this cohort. Several studies have investigated cardiac function and left ventricular architecture in tall athletes, and documented increased septal wall thickness and ventricular mass and dimensions [15, 18,19,20]. Regarding strain analysis by speckle tracking echocardiography Butz et al. found no correlations between strain values and height in athletes, patients with hypertrophic cardiomyopathy and control subjects [4]. However, the influence of left ventricular architecture has been demonstrated to affect strain values [14]. Thus, future studies including tall athletes should aim to recruit a non-athletic control cohort of similar height to reduce the chance of distorted comparisons due to this physical characteristic. Ventricular loading conditions, too, have been shown to influence cardiac strain values [14]; the loading conditions of all participants of the current study were similar, lacking load-influencing co-morbidities and resting in a supine position with no prior physical engagement. A potential error source when assessing apical strain values is apical foreshortening [21]; however, great care was applied during image acquisition and analysis to avoid this phenomenon.
The average septal thickness in the elite athletes of the present study was 0.83 cm ± 0.12 cm and thus within the range of previous observations [22, 23]. As observed in these large athlete cohort studies, septal hypertrophy is generally less pronounced in female elite athletes than in male elite athletes. However, hypertrophy does occur in female athletes, as do pathological conditions such as hypertension or hypertrophic cardiomyopathy. So while the critical question for the sports cardiologist regarding the physiological or pathological nature of borderline septal hypertrophy (ca. 1.5 cm) is a lot more common when caring for male athletes, investigations into the effects on cardiac strain in female athletes with pronounced cardiac adaptations must not be neglected. Also, strain analysis by speckle tracking echocardiography has been demonstrated as a useful tool in the detection of inflammatory cardiac processes [7]. In the day to day care of elite athletes the question of myocarditis arises regularly, thus adding to the necessity to establish strain norm values for all athlete cohorts to allow for strain analysis to be effectively used regarding the diagnosis of this pathology.
Perspective
As mentioned above, the female athlete has been somewhat neglected regarding research of cardiac strain values, their possible alteration due to a high training volume and their utility as a diagnostic tool. The present study adds to the very limited data available of 2D-STE derived left ventricular strain values of female athletes, specifically focusing on female football- and volleyball-athletes with an age- and sex-matched control group. Further research focusing on cardiac strain in female athletes is needed to establish strain analysis by speckle tracking echocardiography as a diagnostic tool across many disciplines of female elite athleticism.
Data availability
The datasets generated and analyzed during the current study are not publicly available to protect the anonymity of the included individuals who-due to their sporting success- are figures of public interest; based on age, height, etc. conclusions regarding identities could be deduced for example by sports journalists. The data are available from the corresponding author on reasonable request. All data is stored for 10 years and contains the raw-data of all measurements described under methods.
References
Prior DL, La Gerche A (2012) The athlete’s heart. Heart 98(12):947–955. https://doi.org/10.1136/heartjnl-2011-301329
Pelliccia A, Adami PE (2017) The female side of the heart: sex differences in athlete’s heart∗. JACC: Cardiovasc Imaging 10(9):973–975. https://doi.org/10.1016/j.jcmg.2016.08.010
Richand V, Lafitte S, Reant P et al (2007) An ultrasound speckle tracking (two-dimensional strain) analysis of myocardial deformation in professional soccer players compared with healthy subjects and hypertrophic cardiomyopathy. Am J Cardiol 100(1):128–132. https://doi.org/10.1016/j.amjcard.2007.02.063
Butz T, van Buuren F, Mellwig KP et al (2011) Two-dimensional strain analysis of the global and regional myocardial function for the differentiation of pathologic and physiologic left ventricular hypertrophy: a study in athletes and in patients with hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 27(1):91–100. https://doi.org/10.1007/s10554-010-9665-5
D’Ascenzi F, Caselli S, Solari M et al (2016) Novel echocardiographic techniques for the evaluation of athletes’ heart: a focus on speckle-tracking echocardiography. Eur J Prev Cardiol 23(4):437–446. https://doi.org/10.1177/2047487315586095
Pelliccia A, Caselli S, Sharma S et al (2018) European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete’s heart. Eur Heart J 39(21):1949–1969
Collier P, Phelan D, Klein A (2017) A test in context: myocardial strain measured by speckle-tracking echocardiography. J Am Coll Cardiol 69(8):1043–1056. https://doi.org/10.1016/j.jacc.2016.12.012
Beaumont A, Grace F, Richards J et al (2017) Left ventricular speckle tracking-derived cardiac strain and cardiac twist mechanics in athletes: a systematic review and meta-analysis of controlled studies. Sports Med 47(6):1145–1170. https://doi.org/10.1007/s40279-016-0644-4
Caselli S, Montesanti D, Autore C et al (2015) Patterns of left ventricular longitudinal strain and strain rate in olympic athletes. J Am Soc Echocardiogr 28(2):245–253. https://doi.org/10.1016/j.echo.2014.10.010
D’Ascenzi F, Pelliccia A, Alvino F et al (2015) Effects of training on LV strain in competitive athletes. Heart. https://doi.org/10.1136/heartjnl-2015-308189
D’Ascenzi F, Pelliccia A, Natali BM et al (2014) Morphological and functional adaptation of left and right atria induced by training in highly trained female athletes. Circ Cardiovasc Imaging 7(2):222–229. https://doi.org/10.1161/CIRCIMAGING.113.001345
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16(3):233–271. https://doi.org/10.1093/ehjci/jev014
Nagueh SF, Smiseth OA, Appleton CP et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 17(12):1321–1360. https://doi.org/10.1093/ehjci/jew082
Voigt J-U, Cvijic M (2019) 2- and 3-Dimensional myocardial strain in cardiac health and disease. JACC: Cardiovasc Imaging 12(9):1849–1863. https://doi.org/10.1016/j.jcmg.2019.01.044
Butz T, van Buuren F, Mellwig KP et al (2010) Systolic and early diastolic left ventricular velocities assessed by tissue Doppler imaging in 100 top-level handball players. Eur J Cardiovasc Prev Rehabil 17(3):342–348
Cappelli F, Toncelli L, Cappelli B et al (2010) Adaptative or maladaptative hypertrophy, different spatial distribution of myocardial contraction. Clin Physiol Funct Imaging 30(1):6–12. https://doi.org/10.1111/j.1475-097X.2009.00896.x
Stefani L, Toncelli L, Di Tante V et al (2008) Supernormal functional reserve of apical segments in elite soccer players: an ultrasound speckle tracking handgrip stress study. Cardiovasc Ultrasound 6(1):14. https://doi.org/10.1186/1476-7120-6-14
Crouse SF, Rohack JJ, Jacobsen DJ (1992) Cardiac structure and function in women basketball athletes: seasonal variation and comparisons with nonathletic controls. Res Q Exerc Sport 63(4):393–401. https://doi.org/10.1080/02701367.1992.10608761
Engel DJ, Schwartz A, Homma S (2016) Athletic cardiac remodeling in US professional basketball players. JAMA Cardiol 1(1):80–87
Malmgren A, Dencker M, Stagmo M et al (2015) Cardiac dimensions and function in female handball players. J Sports Med Phys Fitness 55(4):320–328
Ünlü S, Duchenne J, Mirea O et al (2020) Impact of apical foreshortening on deformation measurements: a report from the EACVI-ASE strain standardization task force. Eur Heart J Cardiovasc Imaging. https://doi.org/10.1093/ehjci/jez189
Pelliccia A (1996) Athlete’s heart in women. JAMA 276(3):211. https://doi.org/10.1001/jama.1996.03540030045030
Finocchiaro G, Dhutia H, D’Silva A et al (2017) Effect of sex and sporting discipline on LV adaptation to exercise. JACC: Cardiovasc Imaging 10(9):965–972. https://doi.org/10.1016/j.jcmg.2016.08.011
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by JZ and IB, analysis was performed by JZ, IB, AS and EG. The first draft of the manuscript was written by JZ and all authors commented on previous versions of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have funding, competing interests or affiliations to disclose.
Ethical approval
All authors are committed to upholding the ethical standards required by the Journal. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee of the German Sport University Cologne, Germany, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zacher, J., Blome, I., Schenk, A. et al. Cardiac adaptations in elite female football- and volleyball-athletes do not impact left ventricular global strain values: a speckle tracking echocardiography study. Int J Cardiovasc Imaging 36, 1085–1096 (2020). https://doi.org/10.1007/s10554-020-01809-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-01809-5