Abstract
Hypertrophic cardiomyopathy (HCM) is a genetic cardiomyopathy with a wide spectrum of clinical manifestations. Patients can be asymptomatic or suffer major adverse events including sudden cardiac death, ventricular arrhythmias, and heart failure. Identification of individuals with HCM who are at risk for these complications remains challenging. While echocardiography remains the mainstay of diagnostic evaluation, cardiac magnetic resonance imaging (CMR) is an important adjunctive diagnostic modality with emerging applications for risk-stratification of adverse events in the HCM population. Although not included in current guidelines for HCM management, there is increasing evidence to support the use of CMR for routine prognostic assessment of HCM patients. In this review we discuss the use of CMR techniques, including late gadolinium enhancement, T1 mapping, and quantification of extracellular volume fraction, for the risk stratification of three major adverse events in HCM: sudden cardiac death, ventricular arrhythmias, and congestive heart failure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypertrophic cardiomyopathy (HCM) is a genetic cardiomyopathy with an autosomal dominant pattern of inheritance affecting approximately 1 in 500 individuals [1]. While half of all patients remain asymptomatic, the rest will experience major adverse events, including ventricular tachyarrhythmias, congestive heart failure (CHF), and sudden cardiac death (SCD) [2]. Identification of individuals at high risk of developing complications is critically important and remains challenging.
Cumulative risk of SCD can be determined using conventional risk factors, including personal history of ventricular tachyarrythmias, family history of SCD, unexplained syncope, maximal left ventricular (LV) thickness ≥ 30 mm, and an abnormal blood pressure response to exercise [3,4,5,6,7]. In 2014, the European Society of Cardiology (ESC) proposed a method incorporating additional risk factors to predict 5-year risk of SCD in HCM patients[8, 9]. This risk prediction tool called “HCM Risk-SCD” was recently validated in a large, international multicenter cohort [10]. Despite the use of HCM Risk-SCD, it is estimated that approximately 0.6% of HCM patients deemed “low-risk” by the tool will experience SCD [11, 7, 3, 12]. Although, HCM Risk-SCD has a moderate-to-high specificity and good negative predictive value, its sensitivity is limited and highlights room for improvement [13]. Importantly, absent from current guidelines for ICD placement is the presence of myocardial fibrosis and other structural abnormalities not included in HCM Risk-SCD. Contrast-enhanced CMR can detect LV apical aneurysms and myocardial fibrosis which are predictive of adverse outcomes [14, 15].
In this review we focus on the use of contrast-enhanced CMR techniques, including late-gadolinium enhancement, T1 mapping, and ECV fraction for detection of myocardial fibrosis risk stratification of three major adverse events in HCM: sudden cardiac death, ventricular arrhythmias, and congestive heart failure. A summary of findings described in this review can be found in Table 1.
Late-gadolinium enhancement
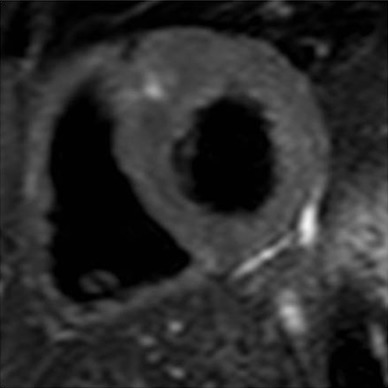
Late gadolinium enhancement (LGE) is a CMR technique optimized to detect focal (replacement) myocardial fibrosis. The method relies on differences in myocardial T1 based on a greater volume of distribution of gadolinium-based contrast media into scarred myocardium versus normal myocardium. LGE was initially used to differentiate between ischemic and non-ischemic cardiomyopathy (NICM) [16], but several groups have also demonstrated its value in differentiation between subtypes of NICM based on patterns of fibrosis [17,18,19]. Up to 70% of HCM patients, including asymptomatic individuals, will exhibit some degree of myocardial fibrosis by LGE [20,21,22] (see Fig. 1). Since the first description of LGE in asymptomatic or minimally symptomatic HCM patients [10], there has been an abundance of literature which supports the use of LGE to predict the risk of SCD, ventricular tachyarrhythmias, and CHF [20, 23,24,25].
Sudden cardiac death
The estimated annual incidence rate of SCD in HCM patients is approximately 1% [8, 26] and is predominately due to ventricular tachycardia/fibrillation (VT/VF), which is mitigated by implantable cardiac defibrillators (ICDs) for primary and secondary prevention [26, 11]. Myocardial fibrosis is a known arrhythmogenic substrate and post-mortem SCD studies have shown the presence of myocardial scar in HCM patients [27, 28].
A meta-analysis of six clinical studies evaluated the relationship between LGE and risk of SCD/aborted SCD in non-high risk HCM patients based on established risk factors [29], and demonstrated that the presence of LGE is associated with increased risk of cardiac death and all-cause mortality, as well as an increased risk of SCD and aborted SCD. Corroborating findings have also been demonstrated by several other groups [30,31,32, 20]. This evidence supports the hypothesis that presence of LGE has predictive value in risk stratification for future SCD of patients with HCM despite risk determined per the HCM-Risk-SCD [29].
Nevertheless, there are potential pitfalls with the use of LGE independently, given that over half of all HCM patients exhibit LGE on CMR [30]. The critical threshold at which LGE imparts an increased risk of SCD has not yet been agreed up on. Currently, if presence of LGE is used as a binary measure to predict future SCD, there would be overestimation of SCD risk and an indication for primary prevention ICDs with uncertain benefit and increased exposure to device-implantation related harm. Chan et al. conducted a multicenter prospective study demonstrating that extensive LGE, defined as ≥ 15% of LV mass, was associated with > twofold increase in SCD risk in asymptomatic, “low risk” patients by conventional risk factors who otherwise would not have been candidates for an ICD. Extensive LGE also conferred a nearly twofold increased risk of SCD in patients with at least one conventional SCD risk factor compared to patients who had conventional risk factors without LGE [30]. Importantly, extent of LGE was also to be a stronger predictor of SCD than individual conventional risk factors. These findings suggest that LGE ≥ 15% of LV mass may be a reasonable threshold for considering a primary prevention ICD in those who fall into an ambiguous risk category per current algorithms. While patients with absence of LGE in the study cohort had lower risk of SCD events, the risk was still non-zero, suggesting that fibrosis alone is not sufficient to fully evaluate risk [33].
Ventricular arrhythmias
NSVT on ambulatory electrocardiogram is a major risk factor for SCD recognized in the current risk stratification algorithm [3]. These arrhythmias likely originate from structurally abnormal myocardium, which often includes areas of myocardial fibrosis. As such, there is growing evidence demonstrating use of LGE, a myocardial fibrosis marker, for the prediction of VT in patients with HCM.
In a retrospective registry study, patients deemed high risk by HCM Risk-SCD had greater extent of LGE compared to low or intermediate risk patients. Extent of LGE was expressed as the percentage of LGE seen in 17 total LV segments. Furthermore, patients who were at low or intermediate risk by HCM Risk-SCD with ≥ 20% LGE were at higher risk of ventricular arrhythmias than those with < 20% LGE. Conversely, in patients who were determined to be high risk with ≤ 20% LGE were at a lower risk of ventricular arrhythmias than those with > 20% LGE [34].
Rubinshtein et al. demonstrated that patients with LGE comprising between 0.4 and 65% of LV were more likely to have frequent NSVT on a 24-h Holter monitor [35]. A subsequent study showed that patients with basal septal fibrosis detected by LGE had VT over 5 times as often as those without scar [36]. Furthermore, Amano et al. demonstrated that patients with VT had significantly greater number of scarred myocardial segments (2.7 vs. 1.3) than patients without VT, again demonstrating quantity of fibrosis is positively associated with risk of ventricular arrhythmias [37]. In a more recent study, LGE signal intensity (LGE-SI) was shown to have importance in predicting VT in HCM. Specifically, intermediate LGE-SI (> 4–6 standard deviations above reference myocardium) was a better predictor of VT than high LGE-SI (> 6 standard deviations above reference myocardium) [38].
Congestive heart failure
Congestive heart failure (CHF) is a common complication of HCM. While CHF in HCM is typically characterized by diastolic dysfunction with an ejection fraction > 75%, a small percentage of patients develop end-stage HCM, characterized by systolic dysfunction [39]. Myocardial fibrosis that occurs in HCM contributes to the myocardial stiffness that leads to diastolic dysfunction. With time, myocardial thinning may occur, subsequently leading to systolic dysfunction. In fact, the degree of myocardial fibrosis has been shown to be inversely proportional to left ventricular ejection fraction [40]. Fibrosis by LGE has been shown to be useful in the investigation of the relationship between myocardial fibrosis and adverse HF-related outcomes in HCM [41,42,43, 25].
In a prospective single center study, O’Hanlon et al. demonstrated that the presence and extent of fibrosis by LGE was associated with a 2.5-fold increase in the development of a composite outcome of HF-related death, HF-related hospitalization, or progression of New York Heart Association class compared to patients without fibrosis. In those with fibrosis, the risk of reaching this endpoint also rose as the percentage of fibrosis present, expressed as a percentage of LV mass, increased. Additionally, they found that presence and extent of LGE were independent predictors of the HF endpoint after multivariate analysis [32].
Additionally, extensive LGE is known to be a strong predictor of end-stage HCM in patients with normal ejection fraction as well as those with low-normal ejection fraction (50–65%), which has implications for the use of LGE in clinical surveillance for progression to end-stage HCM [15, 30]. Moreover, in HCM patients with known systolic dysfunction, extent of LGE has also been shown to be an independent predictor of other major adverse cardiovascular events (MACE), including cardiovascular death, lethal arrhythmia, cardioembolic stroke, and HF-related hospitalization [44].
Native and post-contrast T1 mapping
While LGE sequences are useful to detect focal or regional fibrosis, they are unable to detect diffuse (interstitial) myocardial fibrosis given limitations in spatial resolution. Furthermore, signal intensity on LGE depends on user identification of normal myocardium in order to increase the conspicuity of scarred segments, a process that is inherently flawed in patients with diffuse fibrosis [18, 45, 46]. This has led to the advent of techniques, such as T1 mapping and extracellular collagen volume (ECV) fraction, that more accurately detect diffuse fibrosis. T1 mapping does not rely on the difference in signal intensity between normal and fibrotic myocardium, removing need for healthy reference for measurement and permitting quantification of diffuse fibrosis [47]. T1 mapping relies on individual T1 relaxation times (the time required for excited protons to return from a high-energy state to a low-energy state) on a pixel-level map for measurement. It can be conducted in the intrinsic myocardium (native T1 mapping) or with gadolinium contrast (post-contrast T1 mapping). Native T1 mapping is representative of the combined signal from the myocardium and the extracellular matrix, with longer relaxation times in pathologic states of edema and fibrosis. Exceptions to this pattern include diseases involving lipid- and iron-overload states [18]. Conversely, post-contrast T1 mapping relaxation times are typically shorter, as the paramagnetic effect of gadolinium based contrast agents promote proton relaxation. Post-contrast T1 mapping is more frequently used for detection of diffuse fibrosis due to greater accuracy than native T1 mapping. Because myocardial fibrosis in HCM tends to be diffuse rather than focal, post-contrast T1 mapping is particularly useful in distinguishing the fibrosis of HCM from other non-ischemic cardiomyopathies [47,48,49].
Ventricular arrhythmias
Like regional fibrosis, diffuse fibrosis is also a known arrhythmogenic substrate for ventricular arrhythmias presumably due to the disruption of the electrical conduction between myocyte gap junctions [50]. As an emerging technique, there is little data on the use of T1 mapping for risk stratification of SCD. However, there is a small body of evidence suggesting value in prediction of future ventricular arrhythmias. Chen et al. demonstrated presence of myocardial fibrosis by native T1 mapping in patients with either ischemic or non-ischemic cardiomyopathies was an independent predictor of ventricular arrhythmias in both patient populations [51]. Of the total 138 patients enrolled, only 5 had HCM, limiting the generalizability of these findings to the HCM population. McClellan et al. found that that in HCM subjects, shorter post-contrast ventricular T1 relaxation time was both a significant univariate and multivariate predictor of NSVT detected on either 24 hour-Holter monitor or ICD interrogation. After ROC analysis, they found that a 490 ms threshold was the best performing upper limit post-contrast T1 time for predicting NSVT, as 62% of patients with NSVT in their study had T1 relaxation times < 490 ms, while only 3% with NSVT had T1 relaxation times > 490 ms. Furthermore, they were able to show that post-contrast T1 relaxation times were significantly shorter in patients with aborted SCD, with a significantly larger proportion of patients with aborted SCD having post-contrast T1 times < 440 ms [52]. While it should be noted that post-contrast T1 values may vary due to image timing after contrast administration or gadolinium dosage and rate of clearance [53], these studies provide compelling evidence for future investigation in the HCM population.
Congestive heart failure
Identification of diffuse myocardial fibrosis by T1 mapping has also been implicated in the development of diastolic heart failure in HCM. In a study conducted by Ellims et al., high degrees of diffuse fibrosis identified by shorter post-contrast T1 mapping times were associated with elevated LV filling pressures (suggested by higher E/e’ ratios on echocardiography). Interestingly, the investigators did not find any correlation between the quantity of regional fibrosis by LGE and E/e’ ratios [41]. The same authors also found that patients with significantly shorter post-contrast T1 times reported dyspnea, suggesting that shorter T1 times can be linked with heart failure symptoms [54]. Identification of diffuse fibrosis using T1 mapping may help risk stratify patients that are at higher risk for development of ventricular arrhythmias and may potentially benefit from therapies directed at reducing scar burden to halt progression of diastolic heart failure but data is limited and more research is needed.
Extracellular collagen volume
ECV is another CMR technique used to detect and quantify diffuse myocardial fibrosis. The myocardium is comprised of an intracellular component, intravascular component, and interstitial (extracellular and extravascular) space. ECV measures the extracellular (interstitial) volume fraction and is calculated by using both native and post-contrast T1 mapping values derived from the patient’s blood and myocardium [18]. More specifically, ECV serves as a measure of myocardial tissue remodeling and correlates with the amount of collagen deposition present, calculated using both native and post-contrast T1 mapping times along with the patient’s hematocrit as illustrated below:
Healthy individuals have demonstrated average ECV values of 0.253 ± 0.035 [55]. Compared to post-contrast T1 mapping alone, ECV has better histologic correlation with myocardial fibrosis [18]. With the exception of cardiac amyloid, higher ECV values generally indicate increased collagen deposition, and therefore, larger presence of myocardial fibrosis [18] (see Fig. 2).
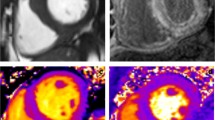
Myocardial mapping in a 63-year-old woman with hypertrophic cardiomyopathy. a Balanced steady state free precession (bSSFP) image demonstrating asymmetric septal hypertrophy with a maximal wall thickness of 22 mm. b Late gadolinium enhancement inversion recovery image with a focus of mid-myocardial enhancement near the inferior right ventricular insertion point (arrow). c, d Native T1 (c) and Extra-cellular volume (ECV) fraction (d) quantitative maps exhibiting areas of patchy increased native T1 and ECV in the inferoseptum consistent with additional sites of interstitial fibrosis
While it has not been studied in the HCM population extensively, ECV fraction has great promise as a prognostic tool for prediction of adverse events in HCM. Avanesov et al. examined the ability of LGE and ECV fraction to predict SCD risk in HCM patients and demonstrated that global ECV, defined as ≥ 34%, was a better predictor of SCD risk compared to LGE (sensitivity 88%, specificity 77%). Additionally, they found that global ECV performed similarly to HCM Risk-SCD and superiorly to LGE and post-contrast T1 mapping in identifying HCM patients with syncope or NSVT. Furthermore, the joint use of global ECV and an HCM Risk-SCD score ≥ 3 had greater accuracy in identifying HCM patients with syncope and NSVT, which presumably could improve identification of candidates for primary prevention ICDs [56]. In general, however, a paucity of data exists on the use of ECV for risk stratification for adverse outcomes in HCM patients and future studies are warranted.
Conclusion
Our knowledge of the use of contrast-enhanced CMR for the risk stratification of HCM patients has grown considerably over the past decade. Late-gadolinium enhancement is a better measure of regional or focal fibrosis, while post-contrast T1 mapping and ECV fraction are identifiers of diffuse fibrosis. The evidence suggests that patients with LGE mass ≥ 15% have increased risk of SCD and fatal ventricular arrhythmias, and should be considered for ICD implantation when risk remains ambiguous based on current risk prediction algorithms. Extensive LGE is seen in patients with end-stage HCM who have developed systolic heart failure. Short post-contrast T1 mapping times inversely correlate with greater degree of diffuse myocardial fibrosis and a cutoff of < 490 ms is the best predictor of NSVT, suggesting these patients could benefit from close monitoring due to conceivably higher risk of SCD. Shortened T1 mapping times also correlate with elevated filling pressures and dyspnea, suggesting that high degree of diffuse fibrosis is linked with greater diastolic dysfunction. Elevated ECV additionally provides quantification of diffuse fibrosis, with global ECV (≥ 34%) shown to be a superior predictor of SCD risk as compared to LGE. Global ECV performs similarly to the HCM Risk-SCD score in identifying HCM patients with syncope and NSVT, and together the use of global ECV and the HCM Risk-SCD score significantly improves accuracy in identifying HCM patients with syncope and NSVT.
While the data is still limited, the evidence provided in this review adds to the justification of a broader application of contrast-enhanced CMR in the screening and surveillance of HCM patients. We recommend that contrast-enhanced CMR should be utilized in patients with inconclusive risk of SCD based on traditional risk factors in order to further risk stratify these patients. If CMR demonstrates high degree of LGE (> 15%), we strongly suggest consideration of ICD implantation and consultation with an electrophysiologist. The evidence regarding the predictive value of T1 mapping times and ECV for ventricular arrhythmias and CHF, respectively, is promising but without robust data to provide definitive recommendations regarding their role in risk stratification. However, we propose that patients with shortened T1 mapping times (< 490 ms) be more closely monitored for ventricular arrhythmias with ambulatory electrocardiographic (ECG) monitoring devices. While exact frequency and duration of monitoring is yet to be determined, we suggest that these higher risk individuals be monitored annually with 1 to 14 day event monitors. Additionally, we suggest that patients with elevated global ECV (≥ 34%) in combination with a high-risk HCM Risk-SCD score be considered for annual ambulatory ECG monitoring for detection of ventricular arrhythmias. Those with a high burden of ventricular arrhythmias should be referred to electrophysiology for consideration of ICD implantation. Currently the evidence regarding the use of CMR in prediction of heart failure in HCM patients is insufficient to provide a recommendation. A summary of these recommendations can be found in Table 2.
While in this review we focus on the utility of contrast-enhanced CMR techniques in HCM, ultimately the prediction of adverse events in HCM likely depends on the use of multiple gadolinium-enhanced CMR-based techniques in addition to other risk factors. For example, anatomical risk factors, such as maximal wall thickness > 30 mm and LV apical aneurysms, in conjunction with high-risk characteristics on CMR using contrast-enhanced techniques, may provide a stronger argument to pursue primary prevention strategies [57]. While the exact contribution of each risk factor remains undetermined, the information presented in this review strongly suggests that contrast-enhanced CMR should be more firmly incorporated into HCM guidelines, as it is a valuable modality for risk stratification and certainly has a role in future clinical decision-making and management algorithms.
References
Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE (1995) Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 92(4):785–789
Rowin EJ, Maron MS, Chan RH, Hausvater A, Wang W, Rastegar H, Maron BJ (2017) Interaction of Adverse Disease Related Pathways in Hypertrophic Cardiomyopathy. Am J Cardiol 120(12):2256–2264. https://doi.org/10.1016/j.amjcard.2017.08.048
Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW (2011) 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 124(24):e783–e831. https://doi.org/10.1161/CIR.0b013e318223e2bd
Christiaans I, van Engelen K, van Langen IM, Birnie E, Bonsel GJ, Elliott PM, Wilde AAM (2010) Risk stratification for sudden cardiac death in hypertrophic cardiomyopathy: systematic review of clinical risk markers. EP Europace 12(3):313–321. https://doi.org/10.1093/europace/eup431
Maki S, Ikeda H, Muro A, Yoshida N, Shibata A, Koga Y, Imaizumi T (1998) Predictors of sudden cardiac death in hypertrophic cardiomyopathy. Am J Cardiol 82(6):774–778
Maron BJ, Ackerman MJ, Nishimura RA, Pyeritz RE, Towbin JA, Udelson JE (2005) Task Force 4: HCM and other cardiomyopathies, mitral valve prolapse, myocarditis, and Marfan syndrome. J Am Coll Cardiol 45(8):1340–1345. https://doi.org/10.1016/j.jacc.2005.02.011
Ommen SR, Gersh BJ (2009) Sudden cardiac death risk in hypertrophic cardiomyopathy. Eur Heart J 30(21):2558–2559. doi:https://doi.org/10.1093/eurheartj/ehp307
Authors/Task Force m, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H, Additional C, O'Mahony C, Guidelines ESCCfP, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol Ç, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, Hasdai D, Ponikowski P, Achenbach S, Alfonso F, Basso C, Cardim NM, Gimeno JR, Heymans S, Holm PJ, Keren A, Kirchhof P, Kolh P, Lionis C, Muneretto C, Priori S, Salvador MJ, Wolpert C, Zamorano JL, Frick M, Aliyev F, Komissarova S, Mairesse G, Smajić E, Velchev V, Antoniades L, Linhart A, Bundgaard H, Heliö T, Leenhardt A, Katus HA, Efthymiadis G, Sepp R, Thor Gunnarsson G, Carasso S, Kerimkulova A, Kamzola G, Skouri H, Eldirsi G, Kavoliuniene A, Felice T, Michels M, Hermann Haugaa K, Lenarczyk R, Brito D, Apetrei E, Bokheria L, Lovic D, Hatala R, Garcia Pavía P, Eriksson M, Noble S, Srbinovska E, Özdemir M, Nesukay E, Sekhri N (2014) 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathyThe Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 35 (39):2733–2779. doi:https://doi.org/10.1093/eurheartj/ehu284
O’mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Gimeno JR, Limongelli G, McKenna WJ (2013) A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J 35(30):2010–2020
O’mahony C, Jichi F, Ommen SR, Christiaans I, Arbustini E, Garcia-Pavia P, Cecchi F, Olivotto I, Kitaoka H, Gotsman I (2018) International External Validation Study of the 2014 European Society of Cardiology Guidelines on Sudden Cardiac Death Prevention in Hypertrophic Cardiomyopathy (EVIDENCE-HCM). Circulation 137(10):1015–1023
Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS, Epstein AE, Almquist AK, Daubert JP, Lawrenz T, Boriani G, Estes NA 3, Favale S, Piccininno M, Winters SL, Santini M, Betocchi S, Arribas F, Sherrid MV, Buja G, Semsarian C, Bruzzi P (2007) Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA 298(4):405–412. https://doi.org/10.1001/jama.298.4.405
Spirito P, Autore C, Formisano F, Assenza GE, Biagini E, Haas TS, Bongioanni S, Semsarian C, Devoto E, Musumeci B, Lai F, Yeates L, Conte MR, Rapezzi C, Boni L, Maron BJ (2014) Risk of sudden death and outcome in patients with hypertrophic cardiomyopathy with benign presentation and without risk factors. Am J Cardiol 113(9):1550–1555. https://doi.org/10.1016/j.amjcard.2014.01.435
Adamczak DM, Oko-Sarnowska Z (2018) Sudden cardiac death in hypertrophic cardiomyopathy. Cardiol Rev 26(3):145–151. https://doi.org/10.1097/crd.0000000000000184
Rowin EJ, Maron MS (2016) The role of cardiac MRI in the diagnosis and risk stratification of hypertrophic cardiomyopathy. Arrhyth Electrophysiol Rev 5(3):197–202. https://doi.org/10.15420/aer.2016:13:3
Olivotto I, Maron MS, Autore C, Lesser JR, Rega L, Casolo G, De Santis M, Quarta G, Nistri S, Cecchi F, Salton CJ, Udelson JE, Manning WJ, Maron BJ (2008) Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 52(7):559–566. https://doi.org/10.1016/j.jacc.2008.04.047
Doltra A, Amundsen BH, Gebker R, Fleck E, Kelle S (2013) Emerging concepts for myocardial late gadolinium enhancement MRI. Curr Cardiol Rev 9(3):185–190. https://doi.org/10.2174/1573403X113099990030
Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O’Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK (2013) Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 309(9):896–908. https://doi.org/10.1001/jama.2013.1363
Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S (2016) Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson 18(1):89. https://doi.org/10.1186/s12968-016-0308-4
Kuruvilla S, Adenaw N, Katwal AB, Lipinski MJ, Kramer CM, Salerno M (2014) Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta-analysis. Circul Cardiovasc Imaging 7(2):250–258. https://doi.org/10.1161/circimaging.113.001144
Weng Z, Yao J, Chan RH, He J, Yang X, Zhou Y, He Y (2016) Prognostic value of LGE-CMR in HCM: a meta-analysis. JACC Cardiovasc imaging 9(12):1392–1402. https://doi.org/10.1016/j.jcmg.2016.02.031
Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, Bonow RO, Judd RM, Kim RJ (2002) Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 40(12):2156–2164
Green JJ, Berger JS, Kramer CM, Salerno M (2012) Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imag 5(4):370–377. https://doi.org/10.1016/j.jcmg.2011.11.021
Ismail TF, Jabbour A, Gulati A, Mallorie A, Raza S, Cowling TE, Das B, Khwaja J, Alpendurada FD, Wage R, Roughton M, McKenna WJ, Moon JC, Varnava A, Shakespeare C, Cowie MR, Cook SA, Elliott P, O’Hanlon R, Pennell DJ, Prasad SK (2014) Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart 100(23):1851–1858. https://doi.org/10.1136/heartjnl-2013-305471
AlJaroudi WA, Flamm SD, Saliba W, Wilkoff BL, Kwon D (2013) Role of CMR imaging in risk stratification for sudden cardiac death. JACC Cardiovasc Imaging 6(3):392–406. https://doi.org/10.1016/j.jcmg.2012.11.011
Green JJ, Berger JS, Kramer CM, Salerno M (2012) Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 5(4):370–377. https://doi.org/10.1016/j.jcmg.2011.11.021
Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, Mahon NG, McKenna WJ (2000) Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol 36(7):2212–2218
Basso C, Thiene G, Corrado D, Buja G, Melacini P, Nava A (2000) Hypertrophic cardiomyopathy and sudden death in the young: pathologic evidence of myocardial ischemia. Human Pathol 31(8):988–998. https://doi.org/10.1053/hupa.2000.16659
Shiozaki AA, Senra T, Arteaga E, Martinelli Filho M, Pita CG, Avila LF, Parga Filho JR, Mady C, Kalil-Filho R, Bluemke DA, Rochitte CE (2013) Myocardial fibrosis detected by cardiac CT predicts ventricular fibrillation/ventricular tachycardia events in patients with hypertrophic cardiomyopathy. J Cardiovasc Comput Tomogr 7(3):173–181. https://doi.org/10.1016/j.jcct.2013.04.002
Briasoulis A, Mallikethi-Reddy S, Palla M, Alesh I, Afonso L (2015) Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: a meta-analysis. Heart 101(17):1406–1411. https://doi.org/10.1136/heartjnl-2015-307682
Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, Udelson JE, Rowin E, Lombardi M, Cecchi F, Tomberli B, Spirito P, Formisano F, Biagini E, Rapezzi C, De Cecco CN, Autore C, Cook EF, Hong SN, Gibson CM, Manning WJ, Appelbaum E, Maron MS (2014) Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 130(6):484–495. doi:https://doi.org/10.1161/circulationaha.113.007094
Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, Udelson JE, Rowin E, Lombardi M, Cecchi F, Tomberli B, Spirito P, Formisano F, Biagini E, Rapezzi C, De Cecco CN, Autore C, Cook EF, Hong SN, Gibson CM, Manning WJ, Appelbaum E, Maron MS (2014) Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 130(6):484–495. https://doi.org/10.1161/circulationaha.113.007094
O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli-Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK (2010) Prognostic Significance of Myocardial Fibrosis in Hypertrophic Cardiomyopathy. J Am Coll Cardiol 56(11):867–874. doi:https://doi.org/10.1016/j.jacc.2010.05.010
Adabag AS, Casey SA, Kuskowski MA, Zenovich AG, Maron BJ (2005) Spectrum and prognostic significance of arrhythmias on ambulatory Holter electrocardiogram in hypertrophic cardiomyopathy. J Am Coll Cardiol 45(5):697–704. https://doi.org/10.1016/j.jacc.2004.11.043
Doesch C, Tulumen E, Akin I, Rudic B, Kuschyk J, El-Battrawy I, Becher T, Budjan J, Smakic A, Schoenberg SO, Borggrefe M, Papavassiliu T (2017) Incremental benefit of late gadolinium cardiac magnetic resonance imaging for risk stratification in patients with hypertrophic cardiomyopathy. Sci Rep 7(1):6336. https://doi.org/10.1038/s41598-017-06533-0
Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, Bos JM, Tajik AJ, Valeti US, Nishimura RA (2009) Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circulation 109:854026
Kwon DH, Smedira NG, Rodriguez ER, Tan C, Setser R, Thamilarasan M, Lytle BW, Lever HM, Desai MY (2009) Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol 54(3):242–249. https://doi.org/10.1016/j.jacc.2009.04.026
Amano Y, Kitamura M, Tachi M, Takeda M, Mizuno K, Kumita S (2014) Delayed enhancement magnetic resonance imaging in hypertrophic cardiomyopathy with Basal septal hypertrophy and preserved ejection fraction: relationship with ventricular tachyarrhythmia. J Comput Assist Tomogr 38(1):67–71. https://doi.org/10.1097/RCT.0b013e3182a2fb01
Appelbaum E, Maron BJ, Adabag S, Hauser TH, Lesser JR, Haas TS, Riley AB, Harrigan CJ, Delling FN, Udelson JE, Gibson CM, Manning WJ, Maron MS (2012) Intermediate-signal-intensity late gadolinium enhancement predicts ventricular tachyarrhythmias in patients with hypertrophic cardiomyopathy. Circul Cardiovasc Imaging 5(1):78–85. https://doi.org/10.1161/circimaging.111.963819
Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey-Bojack S, Manning WJ, Udelson JE, Maron BJ (2006) Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation 114(3):216–225
Jiang M, Wang Z, Su X, Gong X, Pu J, Wu L, Liu C, Yao Q, Kong L, Xu J, He B (2018) The Significance of Interstitial Fibrosis on Left Ventricular Function in Hypertensive versus Hypertrophic Cardiomyopathy. Sci Rep 8(1):9995. https://doi.org/10.1038/s41598-018-27049-1
Ellims AH, Iles LM, Ling L-h, Hare JL, Kaye DM, Taylor AJ (2012) Diffuse myocardial fibrosis in hypertrophic cardiomyopathy can be identified by cardiovascular magnetic resonance, and is associated with left ventricular diastolic dysfunction. J Cardiovasc Magn Reson 14(1):76–76. https://doi.org/10.1186/1532-429X-14-76
Maron BJ, Rowin EJ, Udelson JE, Maron MS (2018) Clinical spectrum and management of heart failure in hypertrophic cardiomyopathy. JACC Heart Fail 6(5):353–363. https://doi.org/10.1016/j.jchf.2017.09.011
Amano Y, Kitamura M, Takano H, Yanagisawa F, Tachi M, Suzuki Y, Kumita S, Takayama M (2018) Cardiac MR imaging of hypertrophic cardiomyopathy: techniques, findings, and clinical relevance. Magn Reson Med Sci 17(2):120–131. https://doi.org/10.2463/mrms.rev.2017-0145
Funada A, Kanzaki H, Noguchi T, Morita Y, Sugano Y, Ohara T, Hasegawa T, Hashimura H, Ishibashi-Ueda H, Kitakaze M, Yasuda S, Ogawa H, Anzai T (2016) Prognostic significance of late gadolinium enhancement quantification in cardiac magnetic resonance imaging of hypertrophic cardiomyopathy with systolic dysfunction. Heart Vessels 31(5):758–770. https://doi.org/10.1007/s00380-015-0670-4
Parsai C, O’Hanlon R, Prasad SK, Mohiaddin RH (2012) Diagnostic and prognostic value of cardiovascular magnetic resonance in non-ischaemic cardiomyopathies. J Cardiovasc Magn Reson 14:54. https://doi.org/10.1186/1532-429x-14-54
Varnava AM, Elliott PM, Sharma S, McKenna WJ, Davies MJ (2000) Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart 84(5):476–482
Jellis CL, Kwon DH (2014) Myocardial T1 mapping: modalities and clinical applications. Cardiovasc Diagn Ther 4(2):126–137. https://doi.org/10.3978/j.issn.2223-3652.2013.09.03
Puntmann VO, Peker E, Chandrashekhar Y, Nagel E (2016) T1 mapping in characterizing myocardial disease: a comprehensive review. Circul Res 119(2):277–299. https://doi.org/10.1161/circresaha.116.307974
Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M (2016) T1 mapping: basic techniques and clinical applications. JACC Cardiovasc Imaging 9(1):67–81. https://doi.org/10.1016/j.jcmg.2015.11.005
Spach MS, Boineau JP (1997) Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol 20(2 Pt 2):397–413
Chen Z, Sohal M, Voigt T, Sammut E, Tobon-Gomez C, Child N, Jackson T, Shetty A, Bostock J, Cooklin M, O’Neill M, Wright M, Murgatroyd F, Gill J, Carr-White G, Chiribiri A, Schaeffter T, Razavi R, Rinaldi CA (2015) Myocardial tissue characterization by cardiac magnetic resonance imaging using T1 mapping predicts ventricular arrhythmia in ischemic and non-ischemic cardiomyopathy patients with implantable cardioverter-defibrillators. Heart Rhythm 12(4):792–801. https://doi.org/10.1016/j.hrthm.2014.12.020
McLellan AJ, Ellims AH, Prabhu S, Voskoboinik A, Iles LM, Hare JL, Kaye DM, Macciocca I, Mariani JA, Kalman JM (2016) Diffuse ventricular fibrosis on cardiac magnetic resonance imaging associates with ventricular tachycardia in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophys 27(5):571–580
Kim PK, Hong YJ, Im DJ, Suh YJ, Park CH, Kim JY, Chang S, Lee H-J, Hur J, Kim YJ, Choi BW (2017) Myocardial T1 and T2 mapping: techniques and clinical applications. Korean J Radiol 18(1):113–131. https://doi.org/10.3348/kjr.2017.18.1.113
Ellims AH, Iles LM, Ling L-h, Chong B, Macciocca I, Slavin GS, Hare JL, Kaye DM, Marasco SF, McLean CA, James PA, du Sart D, Taylor AJ (2014) A comprehensive evaluation of myocardial fibrosis in hypertrophic cardiomyopathy with cardiac magnetic resonance imaging: linking genotype with fibrotic phenotype. Eur Heart J 15(10):1108–1116. https://doi.org/10.1093/ehjci/jeu077
Sado DM, Flett AS, Banypersad SM, White SK, Maestrini V, Quarta G, Lachmann RH, Murphy E, Mehta A, Hughes DA, McKenna WJ, Taylor AM, Hausenloy DJ, Hawkins PN, Elliott PM, Moon JC (2012) Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart 98(19):1436–1441. https://doi.org/10.1136/heartjnl-2012-302346
Avanesov M, Munch J, Weinrich J, Well L, Saring D, Stehning C, Tahir E, Bohnen S, Radunski UK, Muellerleile K, Adam G, Patten M, Lund G (2017) Prediction of the estimated 5-year risk of sudden cardiac death and syncope or non-sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy using late gadolinium enhancement and extracellular volume CMR. Eur Radiol 27(12):5136–5145. https://doi.org/10.1007/s00330-017-4869-x
Maron MS (2012) Clinical utility of cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 14(1):13–13. https://doi.org/10.1186/1532-429X-14-13
Author information
Authors and Affiliations
Contributions
LC and NR generated the idea for this manuscript. NR performed the literature search and drafted this manuscript. LC and BDA prepared the images for the manuscript. SV, JDC, LC critically evaluated and revised this manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to disclose.
Ethical approval
No part of this review has been previously published, and the authors ensure the integrity of this work by accepting full responsibility for all included content.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raiker, N., Vullaganti, S., Collins, J.D. et al. Myocardial tissue characterization by gadolinium-enhanced cardiac magnetic resonance imaging for risk stratification of adverse events in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 36, 1147–1156 (2020). https://doi.org/10.1007/s10554-020-01808-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-01808-6