Abstract
Current guidelines recommend the use of exercise stress echocardiography (ESE) in patients with unexplained dyspnoea. SE was recently reshaped with the ABCDE protocol: A for asynergy, B for B-lines (4-site simplified scan), C for contractile reserve based on force, D for Doppler-based coronary flow velocity reserve (CFVR) in left anterior descending coronary artery; and E for EKG-based heart rate reserve (HRR, defined as peak/rest HR < 1.62). Aim of the study was to define the ESE response in patients with dyspnoea as the main symptom. From the initial population of patients referred in 2018 in a single center for semi-supine ESE, we selected two groups (without history of previous myocardial infarction or coronary revascularization) on the basis of the main presenting symptom: dyspnoea (Group 1, n = 100, 62 men, 63 ± 10 years) or chest pain (Group 2, n = 100, 58 men, age 61 ± 8 years). All underwent ESE with ABCDE protocol. Success rate was 100% for steps A, B, C, E, and 88% for step D. Positivity for A criterion occurred in 56 patients of Group 1 and 24 of Group 2 (p < 0.0001). B-lines positivity (stress > rest for ≥ 2 points) occurred in 40 patients of Group 1 and 28 of Group 2 (p = 0.07). LVCR positivity (< 2.0) occurred in 60 patients of Group 1 and 42 of Group 2 (p < 0.05). A reduced CFVR occurred in 56 of Group 1 and 22 of Group 2 (p < 0.0001). A blunted HRR was present in 44 patients of Group 1 and 22 of Group 2 (p < 0.001). In conclusion, in patients with unexplained dyspnoea, SE with ABCDE protocol is useful to document the cardiac origin of dyspnoea with a comprehensive assessment focused not only on ischemia (A) but also pulmonary congestion (B), myocardial scar or necrosis (C), coronary microvascular dysfunction (D) or chronotropic incompetence (E).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The patient with dyspnoea or exertional fatigue is frequently referred to exercise stress echocardiography (ESE) [1]. In the revised 2019 European Society of Cardiology guidelines for the diagnosis of chronic coronary artery disease, functional testing with ESE is recommended in patients with dyspnoea as the main symptom when availability and local expertise allow [2]. Functional testing with ESE is designed to detect myocardial ischemia through regional wall motion abnormalities (RWMA), but additional abnormalities may involve pulmonary congestion, global LV contractile reserve (LVCR) with cavity dilation, coronary microcirculation dysfunction and chronotropic incompetence, all possible causes or consequences of a dyspnoea of cardiac origin [1,2,3]. Recently, the comprehensive ABCDE protocol has been applied in SE to take the full advantage of the versatility of this technique [4, 5]. It allows to gain a comprehensive view of 5 parameters which can be associated to a dyspnoea of cardiac origin: inducible ischemia (with regional wall motion abnormalities, step A); pulmonary congestion (with B-lines, step B); LVCR based on force (step C); coronary microvascular dysfunction with Doppler-based assessment of coronary flow velocity reserve (CFVR) in mid-distal left anterior descending artery (step D); and EKG-based heart rate reserve (HRR) as a marker of chronotropic incompetence during exercise (step E) [6]. The present study hypothesis is that ABCDE-ESE is feasible and informative in patients with dyspnoea as the primary symptom. To test this hypothesis, we prospectively recruited, in a single center, 200 consecutive patients referred for either chest pain of unknown origin (n = 100) or dyspnoea (n = 100). All patients were studied with the ABCDE-ESE protocol adopted in the SE 2020 study network [7].
Methods
Study population
In this prospective study, we initially screened 875 patients prospectively recruited from December 2016 to March 2019 in a single center following the adoption of SE2020 study protocol and passing of quality control procedures for SE reading [8]. The inclusion criteria were: (1) Age > 18 years; (2) referral for unexplained dyspnoea or chest pain of unknown origin; (3) wall motion imaging by TTE of acceptable quality at rest (< 2 uninterpretable segments); (4) willingness to give their written informed consent allowing scientific utilization of observational data, respectful of privacy rights. The exclusion criteria were: (1) known CAD/history of myocardial infarction/previous myocardial revascularization; (2) severe primary valvular or congenital heart disease; (3) left ventricular outflow tract obstruction or severe mitral insufficiency at rest or peak stress. From the remaining we selected a consecutive group of 100 patients with dyspnoea as the presenting symptom (Group 1) and a consecutive group of 100 patients with chest pain of unknown origin as the presenting symptom (Group 2). Chest pain was either typical or atypical. Typical angina met the following three characteristics: 1—constricting discomfort in the front of the chest or in the neck, jaw, shoulder or arm; 2—precipitated by physical exertion; 3—relieved by rest or nitrates within 5 min. Atypical angina met two of these characteristics [2]. Both dyspnoea and chest pain were exertional symptoms. All patients underwent SE testing as part of a clinically-driven work-up and according to the referring physician's indications. Written informed consent was obtained from all patients before testing. The study protocol was reviewed and approved by the institutional ethics committees as a part of the SE 2020 study (148-Comitato Etico Lazio-1, July 16, 2016; Clinical trials.Gov Identifier NCT 030.49995).
Stress echocardiography
We used commercially available ultrasound machines. All patients underwent comprehensive TTE at rest. Left ventricular volumes were used to calculate EF were measured by modified biplane Simpson’s method [9]. All patients underwent exercise SE according to the protocol recommended by the European Association of Cardiovascular Imaging [10] and American Society of Echocardiography [11]. Exercise was performed with supine bicycle. Imaging was performed at baseline, at an initial workload of 25 watts, at each stage, at peak stress, and in recovery. The workload was increased at increments of 25 watts every 2 min [10, 11].
Criteria for terminating the test were severe chest pain, diagnostic ST-segment shift, excessive blood pressure increase (systolic blood pressure ≥ 240 mmHg, diastolic blood pressure ≥ 120 mmHg), limiting dyspnoea, maximal predicted heart rate, significant arrhythmias. Echocardiographic imaging was performed from parasternal long axis view, short axis view, and apical 4-, and 2-chamber view, using conventional 2-dimensional echocardiography. Step A included assessment of wall motion abnormalities and was performed in all patients. Wall motion score index (WMSI) was calculated in each patient at baseline and peak stress, in a four-point score ranging from 1 (normal) to 4 (dyskinetic) in a 17-segment model of the left ventricle (LV). Step B of protocol included the assessment of B-lines with lung ultrasound and the 4-site simplified scan, from mid-axillary to mid-clavicular lines on the third intercostal space [12]. Step C of protocol included the Force-based assessment of LVCR as the stress/rest ratio of Force, calculated as systolic blood pressure/end-systolic volume [13]. CFVR (step D) was assessed during the standard SE examination using intermittent imaging of wall motion and LAD [5, 14]. Coronary flow in the mid-distal portion of the LAD was imaged from the low parasternal long-axis view and/or modified apical 2-, 3- or 4-chamber view under the guidance of color Doppler flow mapping. All studies were digitally stored to simplify offline reviewing and measurements. At each time point, three optimal profiles of peak diastolic Doppler flow velocities were measured, and the results were averaged.
HRR (step E) was calculated as the peak/rest HR from 12-lead EKG [15].
All steps are performed by the same sonographer/cardiologist with the same transducer. Step A is the standard assessment of RWMA; step B is done in 20 s before the stress and again after the stress with the simplified 4-site scan; step C requires ESV measurements that is a part of the minimum data set of SE (usually employed to measure ejection fraction); step D with Doppler flow imaging in left anterior descending artery is obtained before and during exercise [8, 14]; step E is simply heart rate assessment from EKG.
SE positivity criteria
All positivity criteria were determined a priori. The A criterion was considered positive in presence of stress-induced RWMA (WMSI stress > rest), when at least two adjacent segments of the same vascular territory of the left ventricle showed an increment of at least one point of segmental score during SE [8, 10, 11]. The B criterion was considered positive in presence of stress or rest B-lines ≥ 2 units [8]. The C criterion was considered positive in presence of force-based LVCR ≤ 2.0 [8]. The D criterion was considered positive in presence of coronary flow velocity reserve ≤ 2.0 [8, 14]. The E criterion was considered positive in presence of HRR < 1.69 [15].
As required by stress echo 2020 protocol, all readers had passed the quality control for each of the 4 imaging parameters upstream to starting patient recruitment [5, 16]. SE data were entered in the data bank at the time of testing.
Coronary angiography
Coronary angiography was decided by the referring physician based on symptoms, individual clinical characteristics, and noninvasive imaging results. Obstructive significant CAD was defined by a quantitatively assessed coronary diameter reduction ≥ 50% in the view showing the most severe stenosis. Images were read by experienced invasive cardiologists unaware of the results of SE.
Statistics
Data are expressed as mean ± standard deviation for continuous variables and as numbers (percent) for categorical variables. Continuous variables were compared by paired-samples t test. Proportions were compared by chi-square statistics; Fisher’s exact test was used when appropriate. Differences were considered significant at the 0.05 level when 95% CI did not overlap. The intra- and inter-observer variability of A (WMSI), B (B-lines number), C (ESV), D (peak diastolic flow velocity) and E (HR) was assessed by 2 independent observers (AZ and NZ) who had passed the quality control procedures of SE 2020 and had extensive experience in joint reading in a set of 20 consecutive patients. For intra-observer variability, all analyses were repeated (blinded to the initial results) in 50 consecutive participants more than 30 days after the initial analysis of the same images. For each parameter, interclass concordance coefficient (ICC) was measured and the adjusted coefficient of variation (CV), which is defined as the ratio of the standard deviation and the mean of absolute readings for WMSI, ESV, and peak diastolic flow velocity. A probability value of < 0.05 was considered statistically significant. All statistical calculations were performed using SPSS for Windows, release 18.0 (Chicago, Illinois).
Results
The main clinical, hemodynamic, and rest TTE findings in the 200 patients finally enrolled are shown in Table 1. The 2 groups were comparable for age and gender. Hypertension and ACE-inhibitors therapy was more prevalent in Group 1.
SE findings
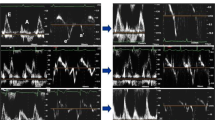
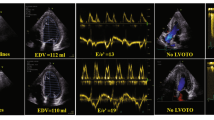
In the overall group of 200 patients, the success rate was 100% for WMSI, 100% for B-lines, 100% for LVCR, 88% for CFVR, 100% for HRR. The intraobserver ICC was 0.93 (95% CI 0.89–0.96, n = 50) for WMSI, 0.96 (95% CI 0.94–0.98, n = 50) for B-lines, 0.98 (95% CI 0.97–0.99, n = 50) for ESV, and 0.98 (95% CI 0.96–0.99, n = 47) for peak diastolic coronary flow velocity. The interobserver ICC was 0.80 (95% CI 0.68–0.88) for WMSI, 0.80 (95% CI 0.68–0.88) for B-lines, 0.90 (95% CI 0.84–0.94) for ESV, and 0.86 (95% CI 0.76–0.92, n = 47) for peak diastolic coronary flow velocity (Fig. 1). The CV was 5.3% for WMSI, 9.9% for ESV, and 6.4% for peak diastolic coronary flow velocity. The main SE findings are reported in Table 2. Examples of an abnormal SE is shown in Fig. 2. The positivity rate was higher in Group 1 compared to Group 2 for the ACDE parameters, separately considered (Fig. 3), and when at least 1 of them was present (Fig. 4).
Feasibility and variability of ACBDE parameters. On the y-axis (%): Success rate, Intra-observer, inter-observer concordance coefficients for each parameter. On the x-axis, from left to right: A (regional wall motion assessment); B (B-lines); C (contractile reserve based on end-systolic volume); D (pulsed-wave Doppler peak diastolic flow); E (heart rate with ECG)
An abnormal ABCDE study. Left column: rest images. Right column, stress images. From top to bottom: A step: abnormal wall motion (septo-apical akinesia during stress (a); B step: 4 B-lines in lung ultrasound during stress (b); C-step: dilated end-systolic volume during stress (c); D-step: blunted increase pulsed-wave Doppler peak diastolic flow—54/34 = 1.59 (d); E step: blunted heart rate with ECG (117/75 = 1.56—d)
Coronary angiographic verification was available in 27 patients, and showed significant CAD in 14/18 patients of Group 1 and 5/9 patients of Group 2. Stress-induced RWMA determining positivity of A criterion were present in 13/14 patients with CAD of Group 1 and 4/5 of Group 2, with a sensitivity of 93% and 80% respectively (p = ns). Stress-induced RWMA determining positivity of A criterion were present in 3/4 patients without CAD of Group 1 and 2/4 of Group 2. Accuracy of A criterion for both groups was 82%, (83% and 67% of Group 1 and Group 2, respectively, p = ns).
Discussion
In cardiac patients, dyspnoea is due to pulmonary congestion as left ventricular dysfunction causes cardiac output to decrease and pulmonary venous pressure to rise, eventually leading to extravasation of fluid into the interstitial space and lung alveoli [1]. Consistently with this interpretation, we observed that in patients with dyspnoea, the ABCDE-ESE protocol is often abnormal for one or more criteria and each one offers important information. RWMA unequivocally indicates presence of inducible ischemia with a highly specific marker of CAD. B abnormality indicates that exercise is accompanied by pulmonary congestion, and proves the cardiac origin of dyspnoea [17, 18]. C abnormality indicates a global LV dysfunction, which can be present with normal WMSI and normal EF-based LV reserve [13]. D can be present in absence of CAD and inducible ischemia, and in this case points to anatomic or functional coronary microvascular disease which is a biomarker and/or cause of heart failure also with preserved ejection fraction [19]. E shows chronotropic incompetence which can be a cause of dyspnoea with or without underlying LV dysfunction or inducible ischemia [20].
Comparison with previous studies
Our study expands previous studies showing that each of the employed parameters can offer useful insight in patients with dyspnoea as the presenting symptom. These patients may frequently show RWMA during stress. Dyspnoea can be an angina equivalent in patients with defective anginal warning system, and is thought to be related to a transient rise in LV end-diastolic pressure caused by ischemia. Previous studies showed that there is a high prevalence of inducible ischemia in aged, overweight patients referred to SPECT testing for dyspnoea [21]. In 1443 patients without known CAD undergoing coronary computed tomography, both dyspnoea and chest pain were equally associated with obstructive CAD [22]. B-lines can be present with inducible ischemia or with normal systolic function in presence of diastolic dysfunction in patients with dyspnoea and normal function [23]. LVCR can be impaired due to inducible ischemia but also in presence of normal EF due to occult reduced systolic reserve [24]. CFVR is impaired in presence of significant CAD but also with normal epicardial coronary arteries for microvascular disease [5]. Finally, an impaired chronotropic reserve is a possible cause of dyspnoea and can be easily detected in patients on or off beta-blockers as a blunted increase in HR during stress, described in CAD but also in HF patients with either reduced or preserved EF [15]. The versatility of functional testing with SE with ABCDE protocol allows to incorporate this comprehensive information in a simple single test.
Clinical implications
The patients with dyspnoea as the presenting symptom is a frequent clinical challenge, with different cardiac and extra-cardiac sources of dyspnoea. The ABCDE protocol allows to unequivocally identify a cardiac origin of dyspnoea in the majority of these patients. Dyspnoea was often an ischemic equivalent, and the documentation of RWMA during stress was corroborated by presence of CAD at coronary angiographic verification.
Study limitations
A significant limitation of the study is that coronary angiographic verification was available only in 27 patients out of 200. However, the study aim was not to assess the diagnostic accuracy of SE, already extensively evaluated in the past for each of the 5 ABCDE steps.
Our results apply only to semi-supine exercise. In patients unable to exercise, pharmacological stress is not recommended, although at least in principle it may offer a similar information, as exercise does, on key variables of ABCDE protocol, including B-lines [12] and chronotropic reserve [24, 25].
We used a simplified index of HRR. The same cutoff value could be calculated with a slightly more complex formula including age and target heart rate: peak HR- rest HR/220—age—rest HR, with cutoff value ≤ 1.62 [6]. Another proposed and prognostically validated approach is to consider chronotropic incompetence in presence of a peak HR < 85% of predicted HR (220—age) it would have been 74% by this method [20]. The prevalence of chronotropic incompetence in our population of dyspnoea patients was 44% with the adopted simplified method, and would have been 60% with the method proposed by Khan et al. [15].
We did not assess E/e′ and tricuspid regurgitant velocity during a dedicated diastolic stress test, as currently recommended in patients with suspected heart failure and preserved ejection fraction. These parameters have recognized feasibility and accuracy limitations during stress. E/e′ is not measurable in 10%, and tricuspid regurgitant velocity jet in 50% of patients during exercise [26]. However, diastolic stress echo is only a third step in the algorithm proposed by the European Society of Cardiology in 2019 in patients with dyspnoea and preserved ejection fraction [26]. At the first step there is functional testing with exercise or conventional SE testing, mainly to exclude alternative causes such as coronary artery disease. This first step can be represented by ABCDE-SE, which allows a more comprehensive assessment to identify specific causes of heart-failure like symptoms, from chronotropic incompetence to myocardial ischemia and microvascular disease. This approach may provide a lot more information than the standard SE [27, 28], and a more comprehensive assessment can be especially fruitful in patients with heterogeneous underlying pathophysiological conditions such as those presenting with dyspnoea.
Conclusions
ABCDE-ESE provides a simple and feasible approach in patients referred for dyspnoea. This approach often provides unequivocal evidence of inducible ischemia and underlying CAD (A abnormality), pulmonary congestion (B abnormality), impaired myocardial reserve due to necrosis or scar (C abnormality), reduced CFVR reserve due to coronary microvascular disease (D abnormality) and chronotropic incompetence (E abnormality). Each of these parameters, and even more their combination, points to a cardiac origin of dyspnoea, and may help in diagnosis which must always integrate clinical, resting transthoracic echocardiography and biomarkers informations, such as left atrial volume, left ventricular hypertrophy and cardiac natriuretic peptides concentration.
Abbreviations
- CAD:
-
Coronary artery disease
- CFVR:
-
Coronary flow velocity reserve
- CV:
-
Coefficient of variation
- EF:
-
Ejection fraction
- ESE:
-
Exercise stress echocardiography
- ESV:
-
End-systolic volume
- HRR:
-
Heart rate reserve
- LV:
-
Left ventricle
- LVCR:
-
Left ventricular contractile reserve
- RWMA:
-
Regional wall motion abnormalities
- SE:
-
Stress echocardiography
- TTE:
-
Transthoracic echocardiography
- WMSI:
-
Wall motion score index
References
Lancellotti P, Pellikka PA, Budts W, Chaudry F, Donal E, Dulgheru R, Edvarsen T, Garbi M, Ha JW, Kane G, Kreeger J, Mertens L, Pibarot P, Picano E, Ryan T, Tsutsui J, Varga A (2016) Recommendations for the clinical use of stress echocardiography in non-ischemic heart disease: joint document of the European Association of Cardiovascular imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging 17:1191–1222
Knuuti J, Wijns W, Saraste A et al (2019) The task force for the diagnosis and management of chronic coronary syndromes of the European Society of cardiology. Eur Heart J 00:1–71
Brubaker PH, Kitzman DW (2011) Chronotropic incompetence. Causes, consequences and management. Circulation 123:1010–1020
Picano E, Ciampi Q, Wierzbowska-Drabik K, Urluescu ML, Morrone D, Carpeggiani C (2018) The new clinical standard of integrated quadruple stress echocardiography with ABCD protocol. Cardiovasc Ultrasound 16:22
Ciampi Q, Zagatina A, Cortigiani L, on behalf of Stress echo 2020 study group et al (2019) Functional, coronary anatomic and prognostic correlates of coronary flow velocity reserve during stress echocardiography. JACC 74:2280–2293
Lauer MS, Okin PM, Larson MG, Evans JC, Levy D (1996) Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation 93:1520–1526
Picano E, Ciampi Q, Citro R, D'Andrea A, Scali MC, Cortigiani L, Olivotto I, Mori F, Galderisi M, Costantino MF, Pratali L, Di Salvo G, Bossone E, Ferrara F, Gargani L, Rigo F, Gaibazzi N, Limongelli G, Pacileo G, Andreassi MG, Pinamonti B, Massa L, Torres MAR, Miglioranza MH, Daros CB, de Castro e Silva Pretto JL, Beleslin B, Djordjevic-Dikic A, Varga A, Palinkas A, Agoston G, Gregori D, Trambaiolo P, Severino S, Arystan A, Paterni M, Carpeggiani C, Colonna P (2017) Stress echo 2020: the international Stress Echo study in ischemic and non-ischemic heart disease. Cardiovasc Ultrasound 15:3
Ciampi Q, Picano E, Paterni M, Borguezan Daros C, Simova I, de Castro e Silva Pretto JL, Scali MC, Gaibazzi N, Severino S, Djordjevic-Dikic A, Kasprzak J, Zagatina A, Varga A, Lowenstein J, Merlo PM, Amor M, Celeutkiene J, Perez J, Di Salvo G, Galderisi M, Mori F, Costantino FM, Massa L, Dekleva M, Quesada Chavez D, Trambaiolo P, Citro R, Colonna P, Rigo F, Torres ARM, Monte I, Stankovic I, Neskovic A, Cortigiani L, Re F, Dodi C, D'Andrea A, Villari B, Arystan A, De Nes M, Carpeggiani C, on behalf of Stress Echo 2020 study group of the Italian Society of Cardiovascular Echography (2017) Quality control of regional wall motion analysis in stress echo 2020. Int J Cardiol 249:479–485
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudsli L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantitation by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28:1–39
Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, Voigt JU, Zamorano JL, on behalf of the European Association of Echocardiography (2008) Stress echocardiography expert consensus statement. European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr 9:415–437
Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG (2007) American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr 20:1021–1024
Scali MC, Zagatina A, Simova I, Zhuravskaya N, Ciampi Q, Paterni M, Marzilli M, Carpeggiani C, Picano E (2017) B-lines with lung ultrasound: the optimal scan technique at rest and during stress. Ultrasound Med Biol 43:2558–2563
Cortigiani L, Huqi A, Ciampi Q, Bombardini T, Bovenzi F, Picano E (2018) Integration of wall motion, coronary flow velocity, and left ventricular contractile reserve in a single test: prognostic value of vasodilator stress echocardiography in patients with diabetes. J Am Soc Echocardiogr 31:692–701
Zagatina A, Zhuravskaya N (2017) The additive prognostic value of coronary flow velocity reserve during exercise echocardiography. Eur Heart J Cardiovasc Imaging 18:1179–1184
Khan MN, Pothier CE, Lauer ME (2005) Chronotropic incompetence as a predictor of death among patients with normal electrocardiogram taking beta-blockers (metoprolol or atenolol). Am J Cardiol 96:1328–1333
Scali MC, Ciampi Q, Picano E, Bossone E, Ferrara F, Citro R et al (2018) Quality control of B-lines analysis in Stress Echo 2020. Cardiovasc Ultrasound 16:20
Scali MC, Cortigiani L, Simionuc A, Gregori D, Marzilli M, Picano E (2017) Exercise-induced B-lines identify worse functional and prognostic stage in heart failure patients with depressed left ventricular function. Eur J Heart Fail 19:1468–1478
Picano E, Scali MC, Ciampi Q, Lichtenstein D (2018) Lung ultrasound for the cardiologist. J Am Coll Cardiol Cardiovasc Imaging 12:381–390
Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan R-S, Beussink-Nelson L, Faxen UL, Fermer ML, Broberg MA, Gan LM, Lund LH (2018) Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction. PROMIS-HFpEF. Eur Heart J 39:3439–3450
Elhendy A, Mahoney DH, Khanderia BK, Burger K, Pellikka PA (2003) Prognostic significance of impairment of heart rate response to exercise: impact of left ventricular function and myocardial ischemia. J Am Coll Cardiol 42:823–830
Balaravi B, Miller TD, Hodge DO, Gibson RJ (2006) The value of stress single photon emission tomography in patients without known coronary artery disease presenting with dyspnoea. Am Heart J 152:551–557
Nakanishi R, Rana JS, Rozanski A et al (2013) Relationship of dyspnoea vs. typical angina to coronary artery disease severity, burden, composition and location on coronary CT angiography. Atherosclerosis 230:61–66
Scali MC, Zagatina A, Ciampi Q, Cortigiani L, Andrea A, Djordjevic-Dikic A, Merlo PM, Lattanzi F, Simova I, Monte I, Dodi C, Kasprzak JD, Galderisi M, Boshchenko A, Rigo F, Varga A, Dekleva M, Re F, de Castro e Silva Pretto JL, Zhuravskaya N, Coviello K, Citro R, Colonna P, Wierzbowska-Drabik K, Carpeggiani C, Picano E, Stress Echo 2020 study group of the Italian Society of Echocardiography, and Cardiovascular Imaging (SIECVI) (2019) The functional meaning of B-profile during stress lung ultrasound. Research letter. JACC Cardiovasc Imaging 12:928–930
Chaowalit N, Mc Cully RB, Callahan MJ, Mookadam F, Bailey KM, Pellikka PA (2006) Outcomes after normal dobutamine stress echocardiography and predictors of adverse events: long-term follow-up of 3014 patients. Eur Heart J 27:3039–3044
Cortigiani L, Carpeggiani C, Landi P, Raciti M, Bovenzi F, Picano E (2019) Usefulness of blunted heart rate reserve as an imaging-independent prognostic predictor during dipyridamole-echocardiography test. Am J Cardiol. https://doi.org/10.1015/j.amjcard.2019.06.017
Pieske B, Tschope C, De Boer RA et al (2019) How to diagnose heart failure with preserved ejection fraction. The HFA-PEFF prognostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Association of Cardiology (ESC). Eur Heart J 40:3297–3317
Mulvagh SL, Mokhtar AT (2019) Coronary flow velocity reserve in stress echocardiography. Time to go with the normal flow? Editorial comment. J Am Coll Cardiol 74:2292–2294
Fuster V (2019) Podcast. Editorial audio summary. JACC 74:2280–2293
Funding
None.
Author information
Authors and Affiliations
Consortia
Contributions
AZ had the original idea and drafted the manuscript; AZ, NZ recruited all patients, critically revised the manuscript for an intellectually important contribution and approved the submitted version; DS critically revised the manuscript for an intellectually important contribution and approved the submitted version; QC is the principal investigator of SE2020, helped to organize the structure of training, contributed to developing the web-based training, and approved the submitted version; CC is responsible for data quality control and reader' certification, performed the data analysis, and helped to draft the manuscript. EP is the study chairman, designed the protocol, organized the content of web-based training, contributed to data analysis and contributed to draft the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A full listing of the members of the Stress Echo 2020 study group can be found at the website http://se2020.altervista.org.
Rights and permissions
About this article
Cite this article
Zagatina, A., Zhuravskaya, N., Shmatov, D. et al. Exercise stress echocardiography with ABCDE protocol in unexplained dyspnoea. Int J Cardiovasc Imaging 36, 823–831 (2020). https://doi.org/10.1007/s10554-020-01789-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-01789-6