Abstract
To evaluate the usefulness of cardiovascular magnetic resonance (CMR) 3D steady state free precession (SSFP) sequence acquired at end-systole (ES) in repaired Tetralogy of Fallot (rToF) patients eligible for percutaneous pulmonary valve implantation (PPVI). Between 2012 and 2018, 78 rToF patients were selected for pulmonary valve replacement (PVR) according to CMR criteria. CMR protocol included 3D-SSFP sequence used to assess the right ventricle outflow tract (RVOT) diameters at three levels (pulmonary valve remnant, mid-portion, bifurcation) in mid-diastole (MD) or ES, RVOT length and coronary artery anatomy. In 20 rToF patients without indications for PVR (controls), 3D SSFP sequence was acquired at both cardiac phases (MD and ES) to evaluate RVOT dimension throughout the cardiac cycle. Invasive balloon sizing was recorded in patients undergoing PPVI. The 3D-SSFP sequence was performed in MD on 39 patients and in ES on other 39, of whom 26 patients met the criteria for PPVI. The latter was unsuccessful in ten patients (38%), mainly due (80% of cases) to significant size discrepancy at PV remnant and bifurcation levels (p = 0.019 and 0.037 respectively) between the measurements by 3D-SSFP in MD and those by the balloon size in systole. Significant RVOT size difference between MD and ES was present at mid-portion and bifurcation levels in the PVR candidate group, and at all three-levels in the control group (all p < 0.001). ES 3D-SSFP sequence is able to quantify RVOT dilation in rToF patients at its maximum expansion, thus improving selection of PPVI candidates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic pulmonary regurgitation (PR) is commonly seen in patients with repaired tetralogy of Fallot (rToF) and may result in right ventricular dilation, biventricular dysfunction, heart failure symptoms, arrhythmias and/or sudden death [1]. Pulmonary valve replacement (PVR) is increasingly used to treat the chronic volume overload due to PR, leading to improvement in functional class and reduction or normalization of right ventricular volumes [2, 3]. PVR can be performed using a transcatheter technique (percutaneous pulmonary valve implantation, PPVI) or surgically (surgical pulmonary valve replacement, SPVR) [2, 4,5,6]. Mortality and morbidity associated with SPVR are low, but not negligible. Percutaneous pulmonary valve implantation (PPVI) is a valid alternative to SPVR in selected cases, given the limited options of diameter sizes available for percutaneous prosthetic valves [7,8,9,10], as well as the impact of the function and anatomy of the right ventricle outflow tract (RVOT) on the procedure [11, 12]. For instance, only recently the Edwards SAPIEN XT valve has been introduced with the bigger size of 29 mm. Even though the procedure is restricted to few cases, its major strength is to eliminate the complications related to cardiopulmonary bypass and sternotomy as well as to shorten the recovery time of the conventional surgical approach. However, even PPVI is an invasive procedure and requires exposure to radiation because a two-step catheterization is needed in these patients (the first for device sizing and placement of a hybrid stent and the second for device deployment) [13]. Therefore, itemized pre-interventional assessment of RVOT morphology and size is fundamental for its success.
At present, cardiovascular magnetic resonance imaging (CMR) is considered the most robust imaging modality for evaluation of ventricular size, systolic function and quantification of pulmonary regurgitation, making it the ideal tool to guide PVR patient selection [14, 15]. Indeed, CMR is an excellent tool to evaluate the right ventricle outflow tract (RVOT) anatomy, pulmonary arteries and aorta, using contrast enhanced MR angiography (CE-MRA) [16]. Furthermore, CE-MRA can now be synchronized with the ecg and this new sequence is replacing the older ungated one for vessel measurements, due to the fact it can be acquired at a specific phase acquisition of the cardiac cycle, thus avoiding to under or overestimate dimensions [17]. However, even gated CE-MRA sequence requires the contrast agent administration, as well as patient cooperation with a perfect apnea and stillness. Recently, the non-contrast enhanced 3D steady-state free precession (SSFP) sequence, triggered by both cardiac and respiratory signals, has been able to provide an accurate evaluation of the dimensions of both thoracic veins and arteries [18,19,20]. Therefore, its use is increasing, particularly in the pediatric age, given that the acquisition can be set at a specific cardiac phase and the influence of respiratory artifacts can be reduced without the need for contrast agent and apnea [19, 20]. Our aim was to evaluate if acquiring non-contrast enhanced 3D SSFP sequence at end-systole (ES) rather than at mid-diastole (MD) was better in order to identify candidates for PPVI, according to the dimension of the prosthetic valves available. In addition, we compared the measurements taken with such sequence with the ones taken at cardiac catheterization, the current gold standard.
Materials and methods
Study population
Between March 2012 and September 2018, among all rToF patients, who underwent CMR assessment for moderate or severe RV dilation evaluated qualitatively at echocardiography, 78 were candidates for PVR based on CMR criteria suggested by the literature [15]. Twenty rToF subjects undergoing CMR with lesser dilation on echocardiography and without PVR indication, were additionally selected as controls. No significant residual RVOT obstruction was present in our series. Patients with a right ventricle-to-pulmonary artery conduit were excluded. All patients were asymptomatic at the time of the study. Patient characteristics and surgical repair data (transannular versus infundibular patch) were collected in all cases.
Cardiovascular magnetic resonance imaging
CMR examinations were performed with a 1.5 T scanner (Achieva, Philips Medical, Best, The Netherlands), using a cardiac 5-channel phased array coil. The study protocol for patients with rToF included retrospectively gated 2D SSFP cine sequences in 2 and 4 chamber views, a short axis stack with whole coverage of both ventricles (10–18 slices; thickness: 5 mm; interslice gap: 2 mm) and RVTO views in the oblique sagittal, coronal and axial planes (thickness 6 mm) to assess for pulmonary expansion during the cardiac cycle. The 3D SSFP navigator sequence was performed with the following parameters: field of view (from 190 × 137 × 77 to 240 × 210 × 151 mm, depending on the patient’s size), acquisition matrix 192 × 144, reconstruction matrix 384 × 384 mm, slice thickness 0.89 mm, acquisition voxel 1.88 × 1.88 × 1.78 mm, reconstruction voxel 0.94 × 0.94 × 0.89 mm, TE = 1.62 ms, TR = 3.2 ms, flip angle = 55°, turbo factor = 28, and sensitivity encoding (SENSE) of 2.2. Respiratory navigator was placed on the right hemi-diaphragm with a 2–4 mm displacement window according to the patient’s BSA. Magnetization preparation pulses included a T2 prepared pulse and a spectral fat-saturation inversion recovery pulse. Data acquisition was performed in the sagittal plane with a navigator acceptance rate of 30–60%, because it is faster than coronal acquisition by reducing the field of view. The phase encoding direction was anterior - posterior. We did not encounter significant de-phasing artifacts in the pulmonary artery region in our experience. The acquisition phase in the PVR group was set at mid-diastole for the patients, who were scanned in earlier years when this was our standard of practice, and at end-systole for the other subjects, who underwent CMR in later years. This change over time was due to some failed percutaneous procedures consequent to underestimation of RVOT measurements taken at CMR using mid-diastole 3D SSFP imaging, whereas the maximum expansibility of the RVOT is at end-systole. In the control group, we used a dual phase option with the acquisition of both mid-diastole and end-systole in one single 3D SSFP sequence in order to analyze the degree of RVOT expansibility during the cardiac cycle. Standard CMR volumes and function were analyzed in all cases.
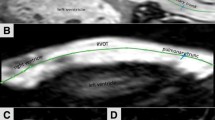
The measurements on the 3D SSFP navigator sequence were performed with a double oblique technique in MPR (Multi-Planar Reconstruction) mode using a commercially available software (Viewforum, Philips Medical, Best, Netherlands). The monitor gray scale was adjusted to avoid image intensity saturation. The RVOT/pulmonary arteries were displayed in three mutually perpendicular planes. Two planes were aligned with the long axis of the vessel and the third was adjusted to provide a cross-section of the vessel. On the latter images, the minimum and maximum diameters were measured at three levels of the RVOT (pulmonary valve remnant, mid-portion, bifurcation). PV remnant was considered at the level of native pulmonary sinus of Valsalva, RVOT mid-portion was assessed when the vessel crossed the aorta in the cross-section and the bifurcation was identified as the most distal segment of the vessel before reaching the pulmonary branches (Fig. 1). The RVOT length between pulmonary valve remnant and bifurcation was also evaluated. Furthermore, the area at level of the pulmonary remnant, mid-portion and bifurcation was calculated considering the cross-section of the vessel as an ellipse, given that the two-vessel major and minor axes are never the same [21] (Fig. 1). The ellipse area A was then calculated multiplying by \(\pi\) the product between the ellipse major semi-axes \(a\) and minor semi-axes \(b\).
All measurements were performed by one expert operator with > 5 years of experience in post-processing. Two weeks after the first evaluation of each patient, the measurements were repeated in all 78 PVR candidates.
Finally, we compared the mean of the two measurements (min and max) at each level (double oblique technique) in diastole or systole (Fig. 2) with the measurement taken at cardiac catheterization in the latero lateral projection in systole in patients selected for PPVI.
PPVI indications and procedure
PPVI has been performed at our Institution since the end of 2011. The procedure was performed in the presence of the following requirements: (1) Appropriate RVOT dimensions and absence of coronary course precluding the procedure as documented by CMR. (2) Patient’s weight no less than 28 kg. Given that at the time of the study the percutaneous valve sizes available were 23 and 26 mm, the hypothesis to perform PPVI was taken into consideration if RVOT dimensions were between 20 and 26 mm and RVOT length was more than 20 mm.
After a right heart catheterization, an angiography in lateral and 15° LAO-30° C view was performed to measure the RVOT in systole. If the measures taken at CMR were comparable to those at angiography, a balloon (generally Z Med, Numed) of appropriate diameter was inflated in the RVOT to check for complete sealing during right ventricular angiography performed at the same time of the inflation and a measurement of RVOT was taken again. Afterwards, any potential compression of the coronary arteries by the stent/valve complex was ruled out by performing coronary angiography during new balloon inflation in the RVOT. In case of incomplete sealing of RVOT or coronary compression, the procedure was interrupted and patients were referred for surgery. Otherwise, PPVI required two steps. The first step involved the placement of a hybrid stent (Andra) in the RVOT to achieve an endothelized surface in order to prepare percutaneous prosthetic valve deployment with a lower risk of migration or dislodgement. The second step was carried out after 2–4 months and consisted in both the positioning of a covered CP stent and the implantation of the prosthetic valve.
Statistical analysis
Results were expressed as the mean value with SD when the data were normally distributed. Otherwise, results were expressed as the median value with range. The Student t-test or ANOVA statistical analysis with Bonferroni test in the case of normally distributed data were performed. Mann Whitney test in the case of non-normally distributed data were used when required. Statistical analysis was performed using SPSS. A p-value of < 0.05 was considered as statistically significant. Spearman’s correlation and Bland–Altman post were performed in order to assess the intraobserver variability.
Results
The sequence was performed in the PVR candidate group in 39 patients in MD and in 39 ones in ES. Twenty-six (33%) out of 78 patients met the criteria for PPVI based on RVOT dimensions evaluated by the 3D SSFP sequence (Fig. 2). The other 52 patients underwent SPVR, without the need for cardiac catheterization beforehand. Out of 26 PPVI procedures, 16 were successful (62%), while the remaining patients were referred for SPVR (Fig. 2). Table 1 shows patient characteristics and CMR data for the PPVI and SPVR group. All 62 patients who needed SPVR showed significantly higher right ventricular end-diastolic volumes and areas at the three pulmonary levels than those with successful PPVI (Table 1). In 8 out of 10 patients (80%) with unsuccessful PPVI, the 3D SSFP sequence was set at mid-diastole and the contraindication to proceed was related to incomplete balloon occlusion of the RVOT secondary to the discrepancy of the measurements between CMR and catheterization. In these patients there was a significant difference between the dimensions taken at CMR and cardiac catheterization at two RVOT levels (remnant and bifurcation), as shown in Table 2. In the other two patients (20%), the sequence was set at end-systole. Of these, one patient had stent dislocation in the right ventricle, while in the other one it was technically impossible to insert the percutaneous pulmonary valve, even though the measurements were favorable. In the group of successful PPVI, the 3DSSFP sequence was set at end-systole in seven patients and at mid-diastole in 9. However, in the nine patients with 3D SSFP imaging set at mid-diastole, there was a statistically significant difference between the measurements taken at CMR and those taken at cardiac catheterization (Table 3), but the initial RVOT values in diastole were so low that they did not go over those required for PPVI when measured at angiography in systole. Instead, in the seven patients with successful PPVI and 3D SSFP sequence set at end-systole there were no statistically significant differences with the measurements taken at cardiac catheterization (Table 4). In all 78 PVR candidates, there was a statistically significant difference between the RVOT area at the level of the mid-portion (p = 0.001) and bifurcation (p = 0.001) between mid-diastole and end-systole, but not the remnant (Table 5). On the basis of the setting of 3D-SSFP (ES and MD), in the two groups of PVR candidate patients there were no statistically significant differences in age at CMR study, BSA, biventricular volumes and function, and pulmonary regurgitation fraction (Table 5). In the control group, a statistically significant difference between the measurements obtained at end-systole and mid-diastole (Table 6) was documented for the area measured at all three RVOT levels (all p < 0.001).
Intraobserver variability
The analysis of the intraobserver variability was performed through test–retest variability of area at level of pulmonary remnant, considering the maximum and minimum dimensions at this level the most technically difficult to take. The analysis showed high concordance of both diastolic and systolic repeated measurements with high spearman rho (both systolic and diastolic rho > 0.99; p < 0.0001) (Fig. 3), as shown by the Bland–Altman plot (Fig. 4).
Discussion
Our paper suggests that CMR using the non-contrast enhanced 3D SSFP sequence set at end-systole, when the right ventricle outflow tract is at its maximum expansion, could be a valid method for the choice of pulmonary valve replacement type (percutaneous versus surgical) in rToF patients treated with transannular or infundibular patch. RVOT measurements for percutaneous prosthesis sizing obtained by using this sequence set at end-systole showed to have excellent correlation with those taken at cardiac catheterization during maximum expansion of the RVOT, the current gold standard. In addition, we documented that RVOT measures taken using 3D SSFP imaging at mid-diastole in patients with unsuccessful PPVI were significantly undersized compared to the dimensions taken with balloon sizing. Furthermore, our study highlighted the presence and importance of the significant expansion of the RVOT during systole compared to diastole, regardless of right ventricular dilatation and/or dysfunction degree. This was confirmed by the use of the dual-phase 3D SSFP sequence acquired at both mid-diastole and end-systole, allowing for evaluation of the entire cardiac cycle. Therefore, setting any 3D sequence by evaluating the RVOT size at end-systole in rToF subjects becomes useful for precise measurements, and even more so in candidates for PVR, in whom there is a greater and different degree of expansion of the RVOT, mainly involving the mid-portion and bifurcation rather than the pulmonary valve remnant, as confirmed by the only other existing CT study [22]. In the PVR group, there were relevant differences in RVOT dimensions measured at the three levels and the right ventricular end-diastolic volume between PPVI and SPVR patients, suggesting that a RVOT area cut-off distinguishing patients suitable and unsuitable for PPVI could be identified in the near future through the use of end-systolic 3D SSFP imaging in a wider rToF population.
Despite its validity, PPVI presents several difficulties, because the function and anatomy of the RVOT, whose morphology results from previous interventions, is unique in each rToF patient. Consequently, PPVI has a realistic chance to be successful only in about 15% of patients referred for PVR [12]. In addition to coronary artery position, RVOT morphology and dilatation during the cardiac cycle as well as its distensibility to “balloon sizing” are the major determinants of PPVI success, as extensively documented in the literature [11, 12, 21,22,23]. The evaluation of all these parameters requires detailed 3D assessment before PPVI. The morphology of the RVOT must be considered mainly in rToF with transannular patch, given that among the 5 RVOT morphology types identified, the pyramidal type that is one of the most common and one of the most frequently associated with this type of surgery, is not suitable for PPVI due to the high risk of device dislocation [11, 12]. The exclusion of RVOT pyramidal shape can be obtained with any 3D imaging, even though it has been precisely documented by patient-specific modeling with finite element method (FEM), using 3D CMR images allowing for virtual device implantation [23]. However, this technique is not easily applicable in clinical practice, and the RVOT shape could also be hypothesized using 3D SSFP imaging alone. Additionally, assessing RVOT expansibility as well as the relationship with the coronary arteries requires a triggered 3D sequence and not just any 3D imaging [24]. Schievano et al. well documented the 3D RVOT/pulmonary arteries deformations during the cardiac cycle and the differences in the measurements of cross-sectional areas between static and dynamic section planes secondary to large displacement and rotation by 4D CT [21]. They also showed how much RVOT diameters, where the potential device could be placed, vary over the cardiac cycle (> 50%) and the real elliptical cross-sectional shape of the pulmonary valve remnant. Our paper not only confirms this evidence, but it also stresses that together with the pulmonary valve remnant, the RVOT mid-portion and bifurcation have an elliptical shape. In our experience, 3D SSFP imaging has shown to be a valid option for complete and precise RVOT assessment at its maximum expansion, due to both its high anatomy resolution and its ability to evaluate any change throughout the cardiac cycle, thus allowing considerable accuracy and reproducibility in RVOT measurements without the need for potentially harmful gadolinium contrast [25, 26] or breath-holding. In addition, the sequence is acquired in the same phase in which the device will be placed, allowing for visualization of the relationship between the coronary arteries and RVOT, and hypothesizing potential compression by the device itself. Therefore, CMR with this sequence may replace CT, the other non-invasive method able to provide 3D ECG-gated images, avoiding radiation dose exposure and iodinated agent contrast administration. This possibility becomes even more important considering that a consistent number of rToF patients under eighteen reach the criteria for PVR. 3D SSFP imaging requires only patient's immobility and could significantly improve patient follow-up, particularly in the pediatric population, allowing to distinguish subjects eligible for PPVI with a high success rate. In our experience, it can guide the decision to perform percutaneous versus surgical treatment with reasonable certainty, avoiding the risks associated with a diagnostic invasive procedure like cardiac catheterization. This is especially relevant considering that PPVI can only be successfully performed in few cases. However, even in the presence of favorable CMR dimensions obtained with 3D SSFP imaging, the compliance of the RVOT and movement of the RVOT/pulmonary trunk junction remains not completely predictable by any 3D imaging, given that RVOT response “to balloon interrogation” can only be evaluated during an interventional procedure [12]. In any case, procedural limitations only rarely occur when pre-interventional 3D SFFP assessment shows adequate RVOT morphology and size.
In conclusion, our experience suggests that CMR using end-systolic 3D SSFP imaging is the only non-invasive and completely harmless method capable of extensively assessing the RVOT in rToF patients treated with transannular and/or infundibular patch before PPVI. Further studies on a wider population could be decisive for the identification of RVOT cut-off values, increasing the ability to predict the possibility of a successful procedure.
Limitations
Because the study was retrospective, the 3D SSFP sequence was not acquired at both mid-diastole and end-systole in all patients who performed CMR and had the criteria for PVR. Moreover, despite being harmless and reproducible, the 3DSSFP sequence needs more time to be performed in comparison with gated-CEMRA. Therefore, it could be difficult to perform in uncooperative children.
References
Martinez RM, Ringewald JM, Fontanet HL, Quintessenza JA, Jacobs JP (2013) Management of adults with Tetralogy of Fallot. Cardiol Young 23:921–932
Burchill LJ, Wald RM, Harris L, Colman JM, Silversides CK (2011) Pulmonary valve replacement in adults with repaired tetralogy of Fallot. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 14:92–97
Ferraz Cavalcanti PE, Sa MP, Santos CA, Esmeraldo IM, de Escobar RR, de Menezes AM, de Azevedo OM, Jr, de Vasconcelos Silva FP, Lins RF, de Lima R C (2013) Pulmonary valve replacement after operative repair of tetralogy of Fallot: meta-analysis and meta-regression of 3,118 patients from 48 studies. J Am Coll Cardiol 62:2227–2243
Frigiola A, Nordmeyer J, Bonhoeffer P (2009) Percutaneous pulmonary valve replacement. Coron Artery Dis 20:189–191
Hawkins JA, Sower CT, Lambert LM, Kouretas PC, Burch PT, Kaza AK, Puchalski MD, Yetman AT (2009) Stentless porcine valves in the right ventricular outflow tract: improved durability? Eur J Cardiothorac Surg 35:600–4; discussion 604–5
Lee C, Lee CH, Kwak JG (2013) Surgical pulmonary valve insertion. Cardiol Young 23:915–920
Demkow M, Ruzyllo W, Biernacka EK, Kalińczuk Ł, Spiewak M, Kowalski M, Sitkowska E, Kuśmierczyk M, Różanski J, Banaś S, Chmielak Z, Hoffman P (2014) Percutaneous Edwards SAPIEN valve implantation for significant pulmonary regurgitation after previous surgical repair with a right ventricular outflow patch. Catheter Cardiovasc Interv 83:474–481
Hascoet S, Acar P, Boudjemline Y (2014) Transcatheter pulmonary valvulation: current indications and available devices. Arch Cardiovasc Dis 107:625–634
Boshoff DE, Cools BL, Heying R, Troost E, Kefer J, Budts W, Gewillig M (2013) Off-label use of percutaneous pulmonary valved stents in the right ventricular outflow tract: time to rewrite the label? Catheter Cardiovasc Interv 81:987–995
Haas NA, Moysich A, Neudorf U, Mortezaeian H, Abdel-Wahab M, Schneider H, De Wolf D, Petit J, Narayanswami S, Laser KT, Sandica E (2013) Percutaneous implantation of the Edwards SAPIEN pulmonic valve: initial results in the first 22 patients. Clin Res Cardiol 102:119–128
Chung R, Taylor AM (2014) Imaging for preintervention planning: transcatheter pulmonary valve therapy. Circ Cardiovasc Imaging 7(1):182–189
Schievano S, Coats L, Migliavacca F, Norman W, Frigiola A, Deanfield J, Bonhoeffer P, Taylor AM (2007) Variations in right ventricular outflow tract morphology following repair of congenital heart disease: implications for percutaneous pulmonary valve implantation. J Cardiovasc Magn Reson 9(4):687–695
Wagner R, Daehnert I, Lurz P (2015) Percutaneous pulmonary and tricuspid valve implantations: An update. World J Cardiol 7(4):167–177
Geva T (2014) Is MRI the preferred method for evaluating right ventricular size and function in patients with congenital heart disease?: MRI is the preferred method for evaluating right ventricular size and function in patients with congenital heart disease. Circ Cardiovasc Imaging 7:190–197
Geva T (2013) Indications for pulmonary valve replacement in repaired tetralogy of fallot: the quest continues. Circulation 128:1855–1857
Geva T, Greil GF, Marshall AC, Landzberg M, Powell AJ (2002) Gadolinium-enhanced 3-dimensional magnetic resonance angiography of pulmonary blood supply in patients with complex pulmonary stenosis or atresia: Comparison with x-ray angiography. Circulation 106:473–478
Groves EM, Bireley W, Dill K, Carroll TJ, Carr JC (2007) Quantitative analysys of ECG-gated high-resolution contrast-enhanced MR angiography of the thoracic aorta. AJR 188:522–528
Chang D, Kong X, Zhou X, Li S, Wang H (2013) Unenhanced steady state free precession versus traditional MR imaging for congenital heart disease. Eur J Radiol 82:1743–1748
Francois CJ, Tuite D, Deshpande V, Jerecic R, Weale P, Carr JC (2008) Unenhanced MR angiography of the thoracic aorta: initial clinical evaluation. AJR Am J Roentgenol 190:902–906
Hussain T, Lossnitzer D, Bellsham-Revell H, Valverde I, Beerbaum P, Razavi R, Bell AJ, Schaeffter T, Botnar RM, Uribe SA, Greil GF (2012) Three-dimensional dual-phase whole-heart MR imaging: clinical implications for congenital heart disease. Radiology 263:547–554
Schievano S, Capelli C, Young C, Lurz P, Nordmeyer J, Owens C, Bonhoeffer P, Taylor AM (2011) Four-dimensional computed tomography: a method of assessing right ventricular outflow tract and pulmonary artery deformations throughout the cardiac cycle. Eur Radiol 21(1):36–45
Schievano S, Taylor AM, Capelli C, Coats L, Walker F, Lurz P, Nordmeyer J, Wright S, Khambadkone S, Tsang V, Carminati M, Bonhoeffer P (2010) First-in-man implantation of a novel percutaneous valve: a new approach to medical device development. EuroIntervention 5(6):745–750
Schievano S, Migliavacca F, Coats L, Khambadkone S, Carminati M, Wilson N, Deanfield JE, Bonhoeffer P, Taylor AM (2007) Percutaneous pulmonary valve implantation based on rapid prototyping of right ventricular outflow tract and pulmonary trunk from MR data. Radiology 242(2):490–497
Ebel S, Gottschling S, Buzan MTA, Grothoff M, Dähnert I, Wagner R, Gräfe D, Lurz P, Gutberlet M, Lücke C (2019) 3D-assessment of RVOT dimensions prior percutaneous pulmonary valve implantation: comparison of contrast-enhanced magnetic resonance angiography versus 3D steady-state free precession sequence. Int J Cardiovasc Imaging. https://doi.org/10.1007/s10554-019-01578-w
Thomsen HS (2016) Nephrogenic systemic fibrosis: a serious adverse reaction to gadolinium—1997-2006-2016. Part 1. Acta Radiol 57(5):515–520
Thomsen HS (2016) Nephrogenic systemic fibrosis: a serious adverse reaction to gadolinium—1997-2006-2016. Part 2. Acta Radiol 57(6):643–648
Acknowledgements
The authors thank Eng. Giovanni Corti for preparing the tables and the picture. The authors express their heartfelt appreciation to the laboratory technicians and nurses of the Department of Imaging.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Leonardi, B., Secinaro, A., Perrone, M.A. et al. Role of cardiovascular magnetic resonance end-systolic 3D-SSFP sequence in repaired tetralogy of Fallot patients eligible for transcatheter pulmonary valve implantation. Int J Cardiovasc Imaging 35, 1525–1533 (2019). https://doi.org/10.1007/s10554-019-01630-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-019-01630-9