Abstract
The aim of this study was to investigate the grading of diastolic dysfunction (DD) in relation to hemodialysis in patients with end stage renal disease (ESRD) on hemodialysis (HD) Cardiovascular disease is prevalent in patients with ESRD and accounts for significant morbidity and mortality. Left ventricular hypertrophy (LVH) is common in ESRD but little is known about the impact of HD on currently recommended grading schemes for DD. Comprehensive echocardiographic data was obtained in consecutive patients with ESRD before (n = 247) and immediately after (n = 239) standard HD regimen. Grading of DD was performed according to current recommendations both pre- and post HD. Prior to HD, DD was classified as present in 83 patients (34%), indeterminate in 51 patients (21%) and absent in 113 patients (45%). Patients with DD at baseline compared to those without were older [67.3 years (13.1) vs. 63.2 (14.3), p = 0.037], were more likely to have diabetic- or hypertensive ESRD (43.4% vs. 35.4%, p = ns) and LVMi was significantly higher [119 g/cm2 (27.5) vs. 103 g/cm2 (24.3), p < 0.001]. After HD [mean HD time = 221 min (27.6), mean ultrafiltration volume = 2 L (1.1)], 39 patients (16%) exhibited sustained DD. These patients were older [69.4 years (14.5) vs. 65.0 years (13.9), p = 0.071], were more likely to have diabetic- or hypertensive ESRD (59% vs. 36%, p = 0.010). Myocardial adverse remodeling was more advanced with higher LVMi [127.4 g/m2 (27.5) vs. 106.5 g/m2 (25.3), p < 0.001], lower LVEF [44.7% (11.0) vs. 54.5% (8.7), p < 0.001] and more impaired GLS [− 13.4% (4.3) vs. − 15.8% (4.0), p = 0.006]. Echocardiographic evaluation of diastolic function in patients with ESRD on HD is critically dependent on timing relative to dialysis. The presence of sustained DD after volume unloading by HD identifies a population of patients with an adverse phenotype of blunted vascular response and severe cardiac remodeling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Patients with end stage renal disease (ESRD) on renal replacement therapy with chronic hemodialysis (HD) have substantial morbidity and mortality with a median survival time of 50 months after initiation of HD [1]. Cardiovascular disease (CVD) is frequent in this patient group with a substantial burden of ischemic heart disease (IHD), valvular heart disease and congestive heart failure (HF) [1]. Utilization of guideline directed medical and interventional treatment is often suboptimal due to substantial co-morbidity and frailty.
Structural alterations of the myocardium dominated by left ventricular (LV) hypertrophy and concentric remodeling are frequent in the early stages of renal impairment [2]. In patients with ESRD both co-morbid conditions such as diabetes and hypertension, as well as the progressive renal dysfunction contribute to LV hypertrophy and fibrosis and ultimately impaired LV filling with or without clinical HF [2,3,4].
Diastolic dysfunction (DD) is highly prevalent in patients with renal dysfunction but estimation of the magnitude of impaired LV filling is critically dependent on loading conditions at the time of echocardiography [5]. Significant shifts in intravascular volume occur during standard HD regimens which could substantially impact the echocardiographic measures used in grading and assessment of DD.
We therefore aimed to characterize baseline diastolic function and importantly, changes in diastolic parameters before and after HD in an unselected consecutive cohort of patients undergoing renal replacement therapy due to ESRD.
Materials and methods
Study population
Consecutive patients 18 years of age or older were included from January through April 2014 at two dialysis centers in the capital region of Denmark. All patients provided written informed consent. The study was approved by the local ethics committee (H-3–2013-098) and by the Danish Data Protection Agency (HIH2013-027). Medical history was obtained from patients and supplemented with chart review. For the purpose of this study, the cause of ESRD was categorized into two groups: (1) patients with hypertensive or diabetic nephropathy, (2) other causes including post glomerulonephritis and polycystic kidney disease amongst others. Classification of the underlying renal pathology was based on thorough chart review and biopsy results when available.
Dialysis treatment
Patients received HD on Gambro Artis™ (Gambro AB, Sweden) machines with large, synthetic high flux filters > 1.6 m2. The filters were either Polyamix® (210H or 170H Gambro Polyflux filters) or Polysulfone (Fresenius FX 100, FX 80, or FX 50 filters, Fresenius Medical Care, Germany). According to international guidelines, the aim of the HD treatment adequacy was to maintain a Kt/V > 1.3/dialysis session, but Kt/V in individual patients was not assessed in connection with this study. Both HD and hemodiafiltration were used in individual patients. Ultrafiltration volume indexed to patient weight was calculated and defined as fluid index (mL/kg).
Transthoracic echocardiography
Transthoracic echocardiography (TTE) was performed within 30 min before and after the dialysis procedure using a Vivid S6 ultrasound machine (GE Vingmed Ultrasound A/S, Horten, Norway). Acquisition of 2D standard views and Doppler images were performed according to EAE/ASE recommendations. Tissue doppler imaging (TDI) was obtained with a pulsed Doppler placed in the lateral and septal, wall at the level of the mitral annulus in the 4-chamber view (Fig. 1).
Echocardiography analyses were performed on EchoPac software (GE Vingmed Ultrasound A/S, Horten, Norway).
Left atrial volume index (LAVi) was determined from the biplane area length method and LVEF was determined using the biplane Simpson model. LV mass index (LVMi) was calculated from the LV linear dimensions in the parasternal view. Volumetric and dimensional measurements of the LV and left atrium were indexed to body surface area when appropriate. All linear dimensions and volumes were indexed to body surface area where appropriate. All volumetric analyses were performed in accordance with EAE/ASE recommendations [6].
Doppler recordings of mitral inflow were performed by placing a 2.5 mm sample volume at the tip of the mitral valve (MV) leaflets during diastole. Peak velocity of early (E) and atrial (A) diastolic filling and MV deceleration time (DT) were measured and E/A-ratio calculated. Peak tricuspid regurgitation (TR) velocity was acquired from continuous wave Doppler. Pulsed wave (PW) TDI recordings were performed at the lateral and medial mitral annulus using a 2.5 mm sample volume with measurements of myocardial peak early velocity (e′). The mean E/e′ ratio was calculated from an average of lateral and medial values of e′ [5].
Global longitudinal strain (GLS) was calculated via a semi-automatic algorithm by speckle tracking in the apical 4-, 2-, and long axis views and using the build in software in EchoPAC as previously reported [7].
Classification of diastolic dysfunction
According to current recommendations, DD should be evaluated using a multiparametric approach rather than relying on a single metric [5]. Patients were classified according to the probability of DD before and after HD. In patients with LVEF > 50% classification was performed according to four metrics: (1) Average E/e′ > 14; (2) Septal e’ velocity < 7 cm/s or lateral e′ velocity < 10 cm/s; (3) TR velocity > 2.8 m/s and (4) LAVi > 34 mL/m2. Patients were characterized as normal diastolic function if < 50% positive; Indeterminate if 50% positive and DD if > 50% positive.
In patients with LVEF < 50% a more complex scheme was used: If E/A ratio < 0.8 and E velocity < 50 cm/s, then LA pressure was deemed normal and DD no more than grade 1 and for the purpose of this study categorized as normal diastolic function. If E/A ratio > 2, then LA pressure was deemed definitely elevated and therefore categorized as DD. In the group with E/A ratio < 0.8 and E velocity > 50 cm/s or 0.8 < E/A ratio < 2, three criteria determined further classification: (1) Average E/e′ > 14; (2) TR velocity > 2.8 m/s and (3) LAVi > 34 mL/m2. When more than one criterion was positive, the patient was classified with DD, else the patients was classified as grade 1 and therefore normal in this study [5].
Statistical analysis
All data are reported as mean ± SD for continuous variables or median or values and percentages for categorical variables. Statistical tests included the by χ2 test for categorical variables and t-test for continuous variables with all tests being two-sided and statistical significance was defined as p < 0.05. Paired measurements were analyzed with paired two-sided t-test. The linear relationship between fluid index and delta values of the paired echocardiographic parameters (post HD–pre HD) was explored in Pearson correlation analysis. To identify baseline clinical and echocardiographic factors independently associated with sustained abnormal LV filling according to DD classification after completion of dialysis, a multiple logistic regression analysis was performed. Candidate variables were entered into the model if p-values < 0.10 in univariable analysis. All analyses were performed using R A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Results
A total of 247 patients (mean age 66 years, 68% male) were included in the study population and had pre-HD echocardiograms. Post-HD echocardiograms were available in 239 patients. Baseline characteristics are presented in Table 1. A total of 99 patients had hypertensive- or diabetic ESRD (n = 50 and n = 49 patients respectively) and 148 patients had either polycystic kidney disease (n = 24), glomerulonephritis (n = 27) or other causes (n = 97). Cardiac co-morbidity was prevalent with 25% of the patients having IHD. There was no significant difference in LVMi or LVEF but GLS, E/e′ and MV E velocity was more impaired in patients with hypertensive- or diabetic ESRD compared to other causes (Table 2).
Prior to HD, DD was classified as present in 83 patients (34%), indeterminate in 51 patients (21%) and absent in 113 patients (45%). There were few statistically significant differences in baseline clinical characteristics according to DD although patients without DD were younger and were more likely to have other causes of ESRD. By definition, diastolic parameters and LVEF were clearly more abnormal in patients with DD however, LVMi was also significantly higher in the DD group [119 g/cm2 (27.5) vs. 103 g/cm2 (24.3), p < 0.001] and GLS more impaired [− 14% (4.2) vs. − 16% (3.4), p = 0.003] (Table 3).
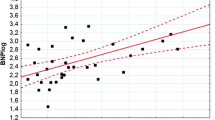
After completion of HD [mean HD time 221 min. (27.6)], mean ultrafiltration volume was 2 L (1.1) and weight reduction was 1.8 kg (1.2) (Table 4). On average, all parameters of diastolic function improved after volume un-loading with HD (Table 4). Reductions in LAVi [35.2 mL/m2 (13.4) vs. 26.6 mL/m2 (12.4), p < 0.001] and prolongation of MV deceleration time (243.9 ms (80.3) vs. 222.6 ms (71.3), p < 0.001) were the most pronounced, whereas E/e′ only changed modestly [12.3 (6.5) vs. 11.7 (7.0), p = 0.015]. There was a statistically significant inverse linear relationship between the volume of fluid removed as assessed by fluid index (mL/kg) and reductions in trans mitral flow velocities ∆E (r = − 0.26, p < 0.001) and ∆A (r = − 0.20, p = 0.007) as well as ∆TR gradient (r = − 0.27, p < 0.001). Changes in myocardial annular velocities ∆e′ (r = − 0.13, p = 0.040), ∆a′ (r = − 0.13, p < 0.056), ∆s’ (r = 0.10, p < 0.142) as well as ∆E/e′ (r = − 0.06, p = 0.410) did not demonstrate a linear relationship with fluid index.
After HD, 39 patients (16%) exhibited sustained DD (Fig. 2) and these patients were older (69.4 years (14.5) vs. 65.0 years (13.9), p = 0.071), were more likely to have diabetic- or hypertensive ESRD (59% vs. 36%, p = 0.010) and exhibited a more blunted hemodynamic response to volume unloading with smaller BP reduction and less increase in HR. Myocardial adverse remodeling was more advanced in patient with sustained DD as evidenced by higher LVMi (127.4 g/m2 (27.5) vs. 106.5 g/m2 (25.3), p < 0.001), lower LVEF (44.7% (11.0) vs. 54.5% (8.7), p < 0.001), more impaired GLS (− 13.4% (4.3) vs. − 15.8% (4.0), p = 0.006) and indices of diastolic function were uniformly more abnormal at baseline (Table 5). In multiple regression analysis including clinical characteristics only, diabetic- or hypertensive ESRD (OR 2.34 (1.13–4.83), p = 0.022) was the only factor independently associated with sustained DD, whereas age [OR 1.02 (1.0–1.06), p = 0.063] and hypertension [OR 1.78 (0.80–3.98), p = 0.162] were only borderline significant. Among baseline echocardiographic measures not directly incorporated in the outcome variable persistent DD (LVEF, GLS and LVMi), only LVEF [OR 0.91 (0.85–0.96), p = 0.001] was significant, whereas GLS [OR 0.99 (0.86–1.14), p = 0.857] and LVMi [OR 1.01 (1.00–1.03), p = 0.12] were not. In multiple logistic regression analysis incorporating baseline measures of diastolic function only E/e′ [OR 1.27 (1.11–1.47), p < 0.001], MV deceleration time [OR 0.98 (0.98–0.99), p = 0.02] and LAVi [OR 1.07 (1.02–1.11), p = 0.004] remained significant, whereas MV E velocity [0.05 (0.00–2.14), p = 0.116] and MV E/A ratio [OR 3.19 (0.87–11.79), p = 0.116] did not.
Discussion
The major findings of the present study can be summarized as follows: (1) In an unselected consecutive cohort of ESRD patients on maintenance dialysis, timing of echocardiography relative to dialysis substantially impacts the likelihood of fulfilling DD criteria according to current guidelines. (2) Sustained DD despite volume unloading is associated with adverse cardiac remodeling and potentially identifies a population of patients with excessive cardiovascular risk.
Diastolic dysfunction in end stage renal disease
The pathway towards DD in patients with ESRD is multifactorial and represents the end result of a complex interaction between renal failure per se and myocardial structural alteration. Afterload on the LV is typically elevated due to sustained increase in systemic vascular resistance (SVR) over decades in patients with hypertensive and/or diabetic ESRD [2]. Activation of the renin-angiotensin system in patients with CKD has well recognized effects on myocardial hypertrophy, fibrosis and remodeling through hyper aldosteronemia [8]. With ensuing CKD progressing towards ESRD, vascular calcification and impaired large vessel compliance, as evidenced by increased aortic pulse wave velocity, further exacerbates the afterload on the LV [9]. Preload is usually elevated in patients with ESRD due to volume expansion in the setting of intermittent fluid overload but also in the context of plasma expansion due to chronic anemia and arteriovenous shunting from dialysis fistulae [10, 11]. In the present study, DD according to current guidelines, was present in 34%, absent in 45% and indeterminate in 21% prior to the initiation of HD. Prevalence of DD in this population varies according to cutoff values of echocardiographic parameters and no studies to our knowledge have included indeterminate diastolic function although this entity, according to our experience, clearly poses a challenge in daily clinical practice. In accordance with prior studies, DD was clearly associated with myocardial remodeling as assessed by increased LVMi, lower LVEF and more impaired GLS [3, 12]. These findings highlight the detrimental effect of increased LV hypertrophy on both active- and passive myocardial relaxation, leading to chronically elevated filling pressure and potentially development overt clinical HF. Volume overload and increased endocardial wall tension activates brain natriuretic peptide synthesis—a powerful marker of adverse clinical outcomes across the spectrum of cardiovascular disease. The role of circulating pro fibrotic markers such as FGF23 as a potentially causative stimulus for cardiac remodeling and vascular maladaptation in CKD patients is increasingly recognized. Whether FGF23 correlated with DD in CKD patients is yet to be determined [13, 14]. Chronic dilatation and fibrotic alterations of the LA in the setting of abnormal LV filling pressure is increasingly recognized as a critical step in LA neurohormonal dysfunction with atrial natriuretic peptide (ANP) synthesis being increasingly defective [15]. In patients with preserved renal function, ANP serves as a powerful stimulant of natriuresis with critical impact on mitigating fluid overload. Whether the interaction between DD through chronic LA remodeling and ANP synthesis impacts differentially on vascular control in the setting of co-existing ESRD remains to be studied.
Diastolic dysfunction before and after hemodialysis
Guideline recommendations at present do not specify the optimal timing of echocardiographic evaluation in patients with ESRD relative to dialysis timing. We found that a significant proportion of patients demonstrated echocardiographic regression of abnormal filling. The volume unloading in HD reduced LV preload as evidenced by a statistically significant reduction in trans mitral filling rate, prolongation of MV deceleration time and interestingly reduction in LAVi. Dilatation of the LA is often perceived as a surrogate of permanently elevated preload and therefore a marker of the chronicity of underlying heart disease [16]. Our data suggest that in patients with ESRD this assumption may not be justified and that fluid status should be taken into consideration. Although fluid removal on average resulted in improved LV filling parameters, we found only relatively weak linear relationships between the absolute volume of fluid removed corrected for patient weight and changes in echocardiographic parameters. Therefore, it is not possible to conclude that changing the intensity of ultrafiltration would translate into incremental improvement in LV filling. Annular myocardial velocities are afterload dependent and inversely correlate with systemic BP and pulse wave velocity [17]. We found no relationship between the magnitude of fluid removal and annular myocardial velocities although mean E/e’ was slightly reduced after dialysis, likely driven by the reduction in E velocity. Prior studies have also shown reduced E velocity after HD in concordance with our study [18] however, there are conflicting data regarding the relationship between e’ values [18, 19] and fluid removal during HD.
A smaller subset of patients demonstrated persistent DD despite volume unloading. This is the first time to the best of our knowledge that such a cardiovascular response is described in patients with ESRD on HD. These patients were older, were more likely to have diabetic- or hypertensive ESRD and exhibited a more blunted vascular response to HD as assessed by a smaller BP reduction and less pronounced increase in HR. This result likely reflects an adverse phenotype of diminished autonomic nervous system function and limited peripheral vascular compliance. There was also evidence of significantly more advanced cardiac remodeling with severe LVH, depressed LVEF in the midrange between 40 and 50% and substantially elevated E/e′ indicative of restrictive LV filling at baseline. Whether this group of patients would benefit from shorter interdialytic intervals or potentially a more aggressive medical therapy is currently unknown.
Limitations
This study was conducted at two major dialysis centers in the Copenhagen area. Results may not be applicable in other geographic regions with different levels of care. The classification of diastolic function was done strictly according to the schematic classification in current guidelines and based on quantitative echocardiographic data. Novel approaches to DD such as left atrial reservoir strain, early diastolic LV strain rate or invasive pressure monitoring were not available in the present study [20, 21]. We did not evaluate clinical outcomes in the present study.
Conclusion
Echocardiographic evaluation of diastolic function in patients with ESRD on HD is critically dependent on timing relative to dialysis. The presence of sustained DD after volume unloading by HD identifies a population of patients with an adverse phenotype of blunted vascular response and severe cardiac remodeling.
Abbreviations
- ESRD:
-
End stage renal disease
- HD:
-
Hemodialysis
- CVD:
-
Cardiovascular disease
- IHD:
-
Ischemic heart disease
- HF:
-
Heart failure
- LVEF:
-
Ventricular ejection fraction
- LVH:
-
Left ventricular hypertrophy
- DD:
-
Diastolic dysfunction
- LA:
-
Left atrium
References
de Jager DJ, Grootendorst DC, Jager KJ et al (2009) Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302:1782–1789
Cerasola G, Nardi E, Palermo A, Mule G, Cottone S (2011) Epidemiology and pathophysiology of left ventricular abnormalities in chronic kidney disease: a review. J Nephrol 24:1–10
McIntyre CW (2009) Effects of hemodialysis on cardiac function. Kidney Int 76:371–375
Whalley GA, Marwick TH, Doughty RN et al (2013) Effect of early initiation of dialysis on cardiac structure and function: results from the Echo Substudy of the IDEAL Trial. Am J Kidney Dis 61:262–270
Nagueh SF, Smiseth OA, Appleton CP et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29:277–314
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–270
Ersboll M, Valeur N, Mogensen UM et al (2013) Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol 61:2365–2373
Di Lullo L, Gorini A, Russo D, Santoboni A, Ronco C (2015) Left Ventricular hypertrophy in chronic kidney disease patients: from pathophysiology to treatment. Cardiorenal Med 5:254–266
Baumann M, Wassertheurer S, Suttmann Y, Burkhardt K, Heemann U (2014) Aortic pulse wave velocity predicts mortality in chronic kidney disease stages 2–4. J Hypertens 32:899–903
Martin LC, Franco RJ, Gavras I et al (2004) Association between hypervolemia and ventricular hypertrophy in hemodialysis patients. Am J Hypertens 17:1163–1169
MacRae JM, Levin A, Belenkie I (2006) The cardiovascular effects of arteriovenous fistulas in chronic kidney disease: a cause for concern? Semin Dial 19:349–352
Jeong JH, Wu PT, Kistler BM et al (2015) The presence and impact of diastolic dysfunction on physical function and body composition in hemodialysis patients. J Nephrol 28:739–747
Naganuma T, Sugimura K, Wada S et al (2002) The prognostic role of brain natriuretic peptides in hemodialysis patients. Am J Nephrol 22:437–444
Vervloet M (2019) Renal and extrarenal effects of fibroblast growth factor 23. Nat Rev Nephrol 15:109–120
Triposkiadis F, Pieske B, Butler J et al (2016) Global left atrial failure in heart failure. Eur J Heart Fail 18:1307–1320
Bakkestrom R, Andersen MJ, Ersboll M et al (2016) Early changes in left atrial volume after acute myocardial infarction Relation to invasive hemodynamics at rest and during exercise. Int J Cardiol 223:717–722
Borlaug BA, Melenovsky V, Redfield MM et al (2007) Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol 50:1570–1577
Hung KC, Huang HL, Chu CM et al (2004) Evaluating preload dependence of a novel Doppler application in assessment of left ventricular diastolic function during hemodialysis. Am J Kidney Dis 43:1040–1046
Assa S, Hummel YM, Voors AA et al (2013) Changes in left ventricular diastolic function during hemodialysis sessions. Am J Kidney Dis 62:549–556
Ersboll M, Andersen MJ, Valeur N et al (2014) Early diastolic strain rate in relation to systolic and diastolic function and prognosis in acute myocardial infarction: a two-dimensional speckle-tracking study. Eur Heart J 35:648–656
Ersboll M, Andersen MJ, Valeur N et al (2013) The prognostic value of left atrial peak reservoir strain in acute myocardial infarction is dependent on left ventricular longitudinal function and left atrial size. Circ Cardiovasc Imaging 6:26–33
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ersbøll, M., Raja, A.A., Warming, P.E. et al. Changes in left ventricular filling parameters before and after dialysis in patients with end stage renal disease. Int J Cardiovasc Imaging 35, 1673–1681 (2019). https://doi.org/10.1007/s10554-019-01619-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-019-01619-4