Abstract
Sigmoid-shaped ventricular septum (SS), a frequently encountered minor abnormality in echocardiographic examinations of the elderly, may have some influence on RV shape. We aimed to determine the influence of SS on the accuracy of the 6 RV linear diameter measurements in the light of three-dimensional echocardiographic (3DE) RV volume. The aorto-septal angle (ASA) was measured in the parasternal long-axis view using two-dimensional echocardiography (2DE) as an index of SS in 70 patients without major cardiac abnormalities who were subdivided into 35 with SS (ASA ≤ 120°) and 35 without SS (NSS). We measured RV end-diastolic volume (RVEDV) using 3DE; in addition, using 2DE, we measured basal RV diameter, mid-cavity diameter, longitudinal diameter and end-diastolic area in the apical four-chamber view; proximal RV outflow tract (RVOT) diameter in the parasternal long-axis view; and proximal and distal RVOT diameters in the parasternal short-axis view. RVEDV did not differ between the SS and NSS groups. The SS group had greater basal RV diameter and proximal and distal RVOT diameters than the NSS group. RV mid-cavity diameter, longitudinal diameter, and end-diastolic area did not differ between the groups. Among the 2DE parameters of RV size, RV end-diastolic area was most strongly correlated with RVEDV (r = 0.67), followed by RV mid-cavity diameter (r = 0.58). When SS is present, the echocardiographic basal RV diameter and RVOT diameters overestimate RV size, and the measurement of RV end-diastolic area and mid-cavity diameter more correctly reflect 3D RV volume.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Several investigators reported the prognostic impact of right ventricular (RV) size and function in patients with idiopathic pulmonary arterial hypertension [1, 2], pulmonary embolism and chronic pulmonary disease [3, 4], and left heart failure [5,6,7]. Until recently, measurement of RV size using two-dimensional (2D) echocardiography had not been standardized because of its complex shape [8, 9]. The American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) published guidelines for the echocardiographic assessment of the right heart size and function [10, 11], which introduced the measurement of 6 RV linear diameters, that is, basal RV inflow diameter, mid-cavity diameter and longitudinal diameter in the apical four-chamber view, proximal RV outflow tract (RVOT) in the parasternal long-axis view, and proximal and distal RVOT diameters in the parasternal short-axis view. The guidelines recommended placing greater importance on basal RV diameter and mid-cavity diameter than on the other diameters [11].
Sigmoid-shaped ventricular septum (SS), a frequently encountered minor abnormality in echocardiographic examinations of the elderly [12,13,14,15], may alter not only left ventricular (LV) shape but also RV shape. In the present study, we aimed to determine the influence of SS on the accuracy of the 6 RV linear diameter measurements as well as the RV end-diastolic area, which was also introduced in the ASE/EACVI guidelines, in the light of three-dimensional echocardiographic (3DE) RV volume.
Subjects and methods
Subjects
Echocardiographic examinations were performed by a single examiner (K.O.) for screening cardiovascular abnormalities in the Kitanodai Clinic from June 2016 to October 2017. We excluded patients with major echocardiographic abnormalities such as obvious LV asynergy, LV systolic dysfunction (LV ejection fraction < 50%), LV hypertrophy (LV mass index > 115 g/m2 for male and > 95 g/m2 for female), signs of pulmonary hypertension [16, 17], cardiac rhythm disturbances such as atrial fibrillation, frequent premature beats and artificial pacing, and inadequate echocardiographic image quality. In addition, we also excluded patients with RV enlargement (RV end-diastolic volume assessed by 3DE > 87 ml/m2 for males and > 74 ml/m2 for females [11]). Ultimately, a total of 70 patients were enrolled in the present study (mean age ± SD, 67.6 ± 11.0 years; range, 22–89 years; 32 males and 38 females).
This study was approved as a retrospective observational study by the Research Ethics Committee of the Faculty of Health Sciences at Hokkaido University. Instead of obtaining informed consent, the objectives and methods of the present study were shared with the public both through our institution’s home page and physical bulletin board; patients who did not wish to participate could request their data be deleted from the study.
Echocardiographic measurements
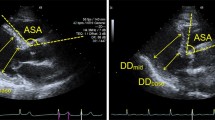
The standard echocardiographic examination was performed in all patients using an ACUSON SC2000 echocardiographic system (Siemens AG, Healthcare Sector, Erlangen, Germany) equipped with a 4V1c transducer (1.25 to 4.5 MHz) in accord with the guidelines of the ASE and EACVI [11]. The aorto-septal angle (ASA) was measured in the end-diastolic parasternal long-axis image as an index of SS (Fig. 1). Following the guidelines, we measured 6 RV linear diameters as follows: basal RV inflow diameter (maximal transverse diameter in the basal third of the RV: RVD1), mid-cavity diameter (transverse diameter in the middle third of the RV, approximately at the level of the papillary muscles: RVD2) and longitudinal diameter (distance from midpoint of the tricuspid annular plane to the apex: RVD3) in the apical four-chamber view; proximal RV outflow tract (RVOT) diameter in the parasternal long-axis view (L-RVOTprox); and proximal and distal RVOT diameters in the parasternal short-axis view (S-RVOTprox and RVOTdistal) [11]. In addition, we also measured RV end-diastolic area in the apical four-chamber view, which was not described in the text page but listed in the Table for the RV measurements of the ASE/EACVI guidelines [11]. A single-beat 3DE image was obtained during end-expiratory breath hold using the same echocardiographic machine with a 4Z1c matrix phased-array (1.5 to 3.5 MHz) transducer. The ultrasound sector size and depth were adjusted to cover the whole RV. After the examination, the RV end-diastolic volume (RVEDV) was measured in each patient using software mounted on the machine (Fig. 2).
Measurements of 6 RV linear diameters and RV end-diastolic area. Proximal right ventricular (RV) outflow tract (RVOT) in the parasternal long-axis view (L-RVOTprox) (A), proximal and distal RVOT diameters in the parasternal short-axis view (S-RVOTprox and RVOTdistal) (B), maximal transverse diameter in the basal third of the RV (RVD1), maximal transverse diameter in the middle third of the RV, approximately at the level of the papillary muscles (RVD2), maximal longitudinal diameter from the tricuspid annulus to the apex (RVD3), and end-diastolic area (RVEDA) in the apical four-chamber view (C), and RV end-diastolic volume measured by single-beat three-dimensional echocardiography (D)
Statistical analysis
The statistical analysis was performed with standard statistical software (IBM SPSS ver. 25 for Windows, IBM Co., Armonk, NY, USA). All numerical data are presented as means ± SD. Differences between the SS and NSS groups were tested by the Student’s t-test. Fisher’s exact test was used when comparing categorical data. Relationships between pairs of parameters were assessed by linear correlation and regression analysis. A stepwise multivariate regression analysis was performed to find independent determinants of the 2D echocardiographic measurements of RV size among multiple parameters. For all statistical tests, a p-value of < 0.05 was considered significant. The reproducibility of RVEDV measured using 3DE was studied in 15 patients randomly selected from the subjects of the present study, and intraclass correlation analysis was performed for the intra-observer comparison (K.O.) and for the inter-observer comparison (K.O. and S.K.).
Results
Patient characteristics and echocardiographic measurements
The patient characteristics and standard echocardiographic parameters of the study subjects are summarized in Table 1. The median value of the ASA was 120 degrees; thus, the study subjects were subdivided into 35 with SS (ASA ≤ 120°) and 35 without SS (ASA > 120°). Other differences between the SS and NSS groups are shown in Table 1. Age was greater in the SS group than in the NSS group (72.2 ± 10.4 vs. 63.0 ± 9.7 years, p < 0.001). Whereas LV end-diastolic diameter, interventricular septal thickness, and LV posterior wall thickness did not differ between the two groups, LV mass index was significantly greater in the SS group than in the NSS group. The prevalence of hypertension, diabetes, and dyslipidemia did not differ between the groups.
Differences in echocardiographic RV size parameters between subjects with SS and those without
The RVEDV and RV end-diastolic area did not differ between SS and NSS groups (Fig. 3). Among the 6 RV linear diameters, L-RVOTprox, S-RVOTprox, RVOTdistal, and RVD1 were greater in the SS group than in the NSS group, whereas RVD2 and RVD3 did not differ between groups (Fig. 4).
Relationships between the 2D echocardiographic RV size parameters and 3D RVEDV
The relationships of the 6 RV linear diameters and RV end-diastolic area to RVEDV are shown in Table 2 and Fig. 5. In the 35 SS patients, RVD2, RVD3, and RV end-diastolic area significantly correlated with RVEDV, but L-RVOTprox, S-RVOTprox, RVOTdistal, and RVD1 did not. On the other hand, in the NSS group, all of the 6 RV linear diameters and RV end-diastolic area significantly and modestly correlated with RVEDV. In all the 70 study patients, all 6 RV linear diameters and the RV end-diastolic area significantly correlated with RVEDV, but the correlations of the L-RVOTprox, S-RVOTprox and RVOTdistal with RVEDV were weak. In each of the above 3 analyses, RV end-diastolic area was most strongly correlated with RVEDV, followed by RVD2.
Impact of the ASA on the 6 RV linear diameters and RV area
The relationships of ASA to the echocardiographic measurements of RV size are summarized in Table 3. ASA was significantly negatively correlated with L-RVOTprox, S-RVOTprox, RVOTdistal, and RVD1, but not with RVD2, RVD3, RV end-diastolic area, or RVEDV.
Stepwise multivariate regression analysis using age, LV mass index, e′, ASA, and RVEDV as explanatory variables revealed that independent determinants for L-RVOTprox, S-RVOTprox and RVD1 were ASA and RVEDV; those for RVOTdistal, RVD2 and RV end-diastolic area were RVEDV, and those for RVD3 were age and RVEDV (Table 4).
Reproducibility of RVEDV measurement using 3DE
The intraclass correlation coefficient was 0.93 (95% confidence interval: 0.80 to 0.98) for the intra-observer comparison and 0.72 (95% confidence interval: 0.36 to 0.90) for the inter-observer comparison.
Discussion
We investigated the influence of SS on the measurements of the 6 RV linear diameters and RV end-diastolic area, which has been recommended by the ASE/EACVI guidelines in the light of 3D echocardiographic RVEDV. In this study, the basal inflow diameter and the outflow tract diameters were greater in subjects with SS than in those without SS despite there being no difference in the RVEDV between the 2 groups. Because all of these diameters significantly negatively correlated with ASA, it is considered that the decrease in ASA (that is, the greater degree of septal deformation due to SS) was the cause of the apparent increase of these diameters. On the other hand, the RV end-diastolic area and mid-cavity diameter were not affected by ASA, and were well correlated with RVEDV. These results suggest that the RV end-diastolic area and the mid-cavity diameter should be preferred to the basal inflow diameter and the outflow diameters for screening RV dilatation in elderly patients.
Impact of sigmoid septum on RV shape
Several reports have indicated that age, gender, and body surface area were determinants of RV size in healthy subjects [18,19,20,21,22]. In the present study, we found that the basal RV inflow and RVOT diameters become greater as the ASA decreased, independent of RV volume, and the ASA was an independent determinant of basal RV diameter and RVOT diameters.
A sigmoid septum (also denoted as ventricular septal bulge or angled aorta) is considered a normal variant or a minor abnormality often encountered in echocardiographic examinations of the elderly [12,13,14,15, 23]. Sometimes, distinguishing between the SS and hypertrophic cardiomyopathy can be problematic because SS can cause an LV outflow tract obstruction [24,25,26,27,28]. It has been also reported that subjects with SS more frequently have hypertension, greater relative wall thickness, and LV diastolic dysfunction [15, 23, 29].
The mechanism by which SS develops has not been fully elucidated. Several investigators have proposed that elongation of the ascending aorta caused by aging and atherosclerosis compresses the LV, causing a decrease in the ASA and an alteration of the shape of the interventricular septum [12, 25, 29]. If the above assumption is true, the ventricular myocardium on the opposite side should also be compressed by the diaphragm and anterior chest wall. Thus, such deformation of the interventricular septum and RV free wall would explain the alteration in RV shape seen in the SS patients of the present study.
Relationship between 2D echocardiographic RV measurement and 3D RVEDV
There are a few reports describing the relationships between the 2D echocardiographic RV measurements recommended by the ASE/EACVI guidelines and the RVEDV derived from cardiovascular magnetic resonance imaging (CMR) [30,31,32]. Lai et al. reported that, in both pediatric and adult patients (4.3‒46.5 years) without RV dilatation, the RV end-diastolic area, RV basal inflow, and mid-cavity diameter significantly correlated with RVEDV while the other linear diameters did not, and in patients with repaired tetralogy of Fallot (3.8‒57.3 years) and those with unrepaired atrial septal defect and/or partially anomalous pulmonary venous connection (0.5‒59.7 years), RV end-diastolic area and RVOT diameters significantly and better correlated with RVEDV than RV inflow diameters [32]. Kim et al. reported that in patients with coronary artery disease (59 ± 13 years), the correlation coefficients with RVEDV was greater for the RV basal diameter (r = 0.70) and proximal RVOT diameter (r = 0.68) than the other RV linear diameters [31]. In all the above studies, the patients were younger than those of the present study. A study by Shiran et al., involving 40 patients including older patients (42 ± 12, 19‒70 years) with different causes of pulmonary hypertension, demonstrated that the correlation with RVEDV by CMR was the best for RV end-diastolic area (r2 = 0.78), followed by RV mid-cavity diameter (r2 = 0.65), and lowest for RV basal inflow diameter (r2 = 0.57) [32]. Their results partially accord with ours, probably because their study subjects included some elderly patients with SS, but they did not analyze the effect of SS on the RV measurements. In the present study, recruiting more elderly subjects, we could clearly demonstrate the effect of SS on the RV linear diameters and the reason for the relatively good correlations of RV end-diastolic area and RV mid-cavity diameter with RVEDV. Our results may contribute to the more accurate evaluation of RV size in routine echocardiographic practice in elderly patients.
Validity of 3D echocardiographic RV volume measurement
CMR has been used as a standard method to measure RV volume and assess RV geometry [9, 33]. However, CMR cannot be performed in patients with mechanical prosthesis, pacemakers, or metallic devices, or those who are extremely obese or have difficulty staying in small physical spaces. Recently, RV volume analysis software using 3DE has emerged, and it has been reported that the RV volume measured using 3DE is comparable with those using CMR [34, 35]. Thus, we used 3DE RV volume as the standard for RV size in the present study. However, only some high-end echocardiographic machines are equipped with 3DE, and the procedure may be time-consuming for routine use. Thus, at present, 2D echocardiography remains the main screening tool for assessing RV size.
Clinical importance of assessing RV size
RV dilatation is reported to be a powerful predictor of poor survival in patients with acute pulmonary embolism [4], idiopathic pulmonary arterial hypertension [1, 2], and chronic pulmonary disease [3]. In addition, assessing RV size is also useful to estimate the extent of RV volume overload due to tricuspid regurgitation [36, 37] and congenital heart diseases such as atrial septal defect and pulmonary regurgitation after the repair of tetralogy of Fallot [36, 38]. RV dilatation can be caused by post-capillary pulmonary hypertension due to left heart failure [39], and has been reported to be useful in predicting outcomes of patients with left heart failure [40]. Thus, an accurate estimation of RV size is important in the management of patients with a wide range of cardiovascular diseases.
The present study also showed an increase in RVOT diameters in subjects with SS. In patients with arrhythmogenic right ventricular cardiomyopathy (ARVC), increased RV size especially in RVOT is considered a characteristic finding. Task Force guidelines for the diagnosis of the ARVC listed enlarged RVOT diameters (L-RVOTprox ≥ 33 mm or S-RVOTprox ≥ 36 mm) as one of the major criteria of ARVC [41]. However, in the present study, L-RVOTprox ≥ 33 mm was seen in 22 of the 35 SS patients (63%) and S-RVOTprox ≥ 36 mm in 8 of the 35 (23%) SS patients. When detecting increased RVOT diameters in echocardiographic examination of elderly subjects, the influence of the SS should be considered before attributing the findings to true RV dilatation due to ARVC.
Limitations
There are several limitations in the present study. First, CMR was not performed and we could not assess the precise RV geometry and the relationship to the surrounding structures. Second, the data collection of the present study was performed at a single center and the study population was relatively small. A study recruiting a greater number of patients may be needed to establish the exact methods to measure the RV size in routine echocardiographic examination.
References
Ghio S, Pazzano AS, Klersy C, Scelsi L, Raineri C, Camporotondo R et al (2011) Clinical and prognostic relevance of echocardiographic evaluation of right ventricular geometry in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol 107:628–632
Baggen VJ, Leiner T, Post MC, van Dijk AP, Roos-Hesselink JW, Boersma E et al (2016) Cardiac magnetic resonance findings predicting mortality in patients with pulmonary arterial hypertension: a systematic review and meta-analysis. Eur Radiol 26:3771–3780
Burgess MI, Mogulkoc N, Bright-Thomas RJ, Bishop P, Egan JJ, Ray SG (2002) Comparison of echocardiographic markers of right ventricular function in determining prognosis in chronic pulmonary disease. J Am Soc Echocardiogr 15:633–639
Frémont B, Pacouret G, Jacobi D, Puglisi R, Charbonnier B, de Labriolle A (2008) Prognostic value of echocardiographic right/left ventricular end-diastolic diameter ratio in patients with acute pulmonary embolism: results from a monocenter registry of 1,416 patients. Chest 133:358–362
Meyer P, Filippatos GS, Ahmed MI, Iskandrian AE, Bittner V, Perry GJ et al (2010) Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation 121:252–258
Mohammed SF, Hussain I, AbouEzzeddine OF, Takahama H, Kwon SH, Forfia P et al (2014) Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation 130:2310–2320
Ghio S, Temporelli PL, Klersy C, Simioniuc A, Girardi B, Scelsi L et al (2013) Prognostic relevance of a non-invasive evaluation of right ventricular function and pulmonary artery pressure in patients with chronic heart failure. Eur J Heart Fail 15:408–414
Haddad F, Hunt SA, Rosenthal DN, Murphy J (2008) Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 117:1436–1448
Surkova E, Muraru D, Iliceto S, Badano LP (2016) The use of multimodality cardiovascular imaging to assess right ventricular size and function. Int J Cardiol 214:54–69
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K et al (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23:685–713
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28:1–39
Goor D, Lillehei CW, Edwards JE (1969) The “sigmoid septum,” variation in the contour of the left ventricular outlet. Am J Roentgenol 107:366–376
Waller BF (1988) The old-age heart: normal aging changes which can produce or mimic cardiac disease. Clin Cardiol 11:513–517
Funabashi N, Umazume T, Takaoka H, Kataoka A, Ozawa K, Uehara M et al (2013) Sigmoid shaped interventricular septum exhibit normal myocardial characteristics and has a relationship with aging, ascending aortic sclerosis and its tilt to left ventricle. Int J Cardiol 168:4484–4488
Okada K, Mikami T, Kaga S, Nakabachi M, Abe A, Yokoyama S et al (2014) Decreased aorto-septal angle may contribute to left ventricular diastolic dysfunction in healthy subjects. J Clin Ultrasound 42:341–347
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A et al (2016) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37:67–119
Hioka T, Kaga S, Mikami T, Okada K, Murayama M, Masauzi N et al (2017) Overestimation by echocardiography of the peak systolic pressure gradient between the right ventricle and right atrium due to tricuspid regurgitation and the usefulness of the early-diastolic transpulmonary valve pressure gradient for estimating pulmonary artery pressure. Heart Vessels 32:833–842
Tamborini G, Marsan NA, Gripari P, Maffessanti F, Brusoni D, Muratori M et al (2010) Reference values for right ventricular volumes and ejection fraction with real-time three-dimensional echocardiography: evaluation in a large series of normal subjects. J Am Soc Echocardiogr 23:109–115
Ogunyankin KO, Liu K, Lloyd-Jones DM, Colangelo LA, Gardin JM (2011) Reference values of right ventricular end-diastolic area defined by ethnicity and gender in a young adult population: the CARDIA study. Echocardiography 28:142–149
D’Oronzio U, Senn O, Biaggi P, Gruner C, Jenni R, Tanner FC et al (2012) Right heart assessment by echocardiography: gender and body size matters. J Am Soc Echocardiogr 25:1251–1258
Willis J, Augustine D, Shah R, Stevens C, Easaw J (2012) Right ventricular normal measurements: time to index? J Am Soc Echocardiogr 25:1259–1267
Henein M, Waldenström A, Mörner S, Lindqvist P (2014) The normal impact of age and gender on right heart structure and function. Echocardiography 31:5–11
Gaudron PD, Liu D, Scholz F, Hu K, Florescu C, Herrmann S et al (2016) The septal bulge: an early echocardiographic sign in hypertensive heart disease. J Am Soc Hypertens 10:70–80
Dalldorf FG, Willis PW (1985) Angled aorta (“sigmoid septum”) as a cause of hypertrophic subaortic stenosis. Hum Pathol 16:457–462
Krasnow N (1997) Subaortic septal bulge simulates hypertrophic cardiomyopathy by angulation of the septum with age, independent of focal hypertrophy. An echocardiographic study. J Am Soc Echocardiogr 10:545–555
Kobayashi S, Sakai Y, Taguchi I, Utsunomiya H, Shiota T (2018) Causes of increased pressure gradient through the left ventricular outflow tract: a West Coast experience. J Echocardiogr 16:34–41
Ranasinghe I, Yeoh T, Yiannikas J (2011) Negative ionotropic agents for the treatment of left ventricular outflow tract obstruction due to sigmoid septum and concentric left ventricular hypertrophy. Heart Lung Circ 20:579–586
Canepa M, Pozios I, Vianello PF, Ameri P, Brunelli C, Ferrucci L et al (2016) Distinguishing ventricular septal bulge versus hypertrophic cardiomyopathy in the elderly. Heart 102:1087–1094
Swinne CJ, Shapiro EP, Jamart J, Fleg JL (1996) Age-associated changes in left ventricular outflow tract geometry in normal subjects. Am J Cardiol 78:1070–1073
Lai WW, Gauvreau K, Rivera ES, Saleeb S, Powell AJ, Geva T (2008) Accuracy of guideline recommendations for two-dimensional quantification of the right ventricle by echocardiography. Int J Cardiovasc Imaging 24:691–698
Kim J, Srinivasan A, Seoane T, Di Franco A, Peskin CS, McQueen DM et al (2016) Echocardiographic linear dimensions for assessment of right ventricular chamber volume as demonstrated by cardiac magnetic resonance. J Am Soc Echocardiogr 29:861–870
Shiran H, Zamanian RT, McConnell MV, Liang DH, Dash R, Heidary S et al (2014) Relationship between echocardiographic and magnetic resonance derived measures of right ventricular size and function in patients with pulmonary hypertension. J Am Soc Echocardiogr 27:405–412
Valsangiacomo Buechel ER, Mertens LL (2012) Imaging the right heart: the use of integrated multimodality imaging. Eur Heart J 33:949–960
Park JB, Lee SP, Lee JH, Yoon YE, Park EA, Kim HK et al (2016) Quantification of right ventricular volume and function using single-beat three-dimensional echocardiography: a validation study with cardiac magnetic resonance. J Am Soc Echocardiogr 29:392–401
Grapsa J, O’Regan DP, Pavlopoulos H, Durighel G, Dawson D, Nihoyannopoulos P (2010) Right ventricular remodelling in pulmonary arterial hypertension with three-dimensional echocardiography: comparison with cardiac magnetic resonance imaging. Eur J Echocardiogr 11:64–73
Venkatachalam S, Wu G, Ahmad M (2017) Echocardiographic assessment of the right ventricle in the current era: Application in clinical practice. Echocardiography 34:1930–1947
Dreyfus GD, Martin RP, Chan KM, Dulguerov F, Alexandrescu C (2015) Functional tricuspid regurgitation: a need to revise our understanding. J Am Coll Cardiol 65:2331–2336
Warnes CA (2009) Adult congenital heart disease. J Am Coll Cardiol 54:1903–1910
Dandel M, Hetzer R (2016) Echocardiographic assessment of the right ventricle: Impact of the distinctly load dependency of its size, geometry and performance. Int J Cardiol 221:1132–1142
Bourantas CV, Loh HP, Bragadeesh T, Rigby AS, Lukaschuk EI, Garg S et al (2011) Relationship between right ventricular volumes measured by cardiac magnetic resonance imaging and prognosis in patients with chronic heart failure. Eur J Heart Fail 13:52–60
Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA et al (2010) Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 121:1533–1541
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okada, K., Kaga, S., Tsujita, K. et al. Right ventricular basal inflow and outflow tract diameters overestimate right ventricular size in subjects with sigmoid-shaped interventricular septum: a study using three-dimensional echocardiography. Int J Cardiovasc Imaging 35, 1211–1219 (2019). https://doi.org/10.1007/s10554-019-01536-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-019-01536-6