Abstract
The present study investigated the changes of biventricular mechanics at rest and during exercise and examined the association between exercise capacity and biventricular mechanics and functional reserve in nonobstructive hypertrophic cardiomyopathy (NHCM) patients. A total of 50 NHCM patients and 25 controls were consecutively recruited for this study. Using echocardiography and two-dimensional speckle-tracking imaging, an experienced echocardiographer determined the following indices: RV free wall longitudinal strain (RVFWLS), LV global longitudinal strain (LVGLS), strain rate (SR), and functional reserve of strain values. We also investigated the relationships between biventricular mechanics and exercise capacity using metabolic equivalents (METs). NHCM patients had lower RVFWLS, LVGLS, systolic SR, early diastolic SR, and systolic and diastolic reserve during exercise compared to controls. An association of biventricular mechanics (LVGLS, RVFWLS) with exercise capacity at rest and during exercise was established. Multivariable logistic regression revealed that RVFWLS and LVE/e′ during exercise (RVFWLS-exe, E/e′-exe) were independent predictors of exercise intolerance. Receiver operating characteristic curve analysis indicated that LVE/e′-exe had a higher area under the curve for predicting exercise intolerance in NHCM patients. In hierarchical analysis, RVFWLS-exe provided an incremental predictive value of exercise intolerance over LVGLS during exercise (LVGLS-exe) and LVE/e′-exe. LVE/e′-exe also changed incrementally compared to LVGLS-exe and RVFWLS-exe. NHCM patients have decreased biventricular mechanics at rest and during exercise and impaired biventricular functional reserve, and biventricular mechanics are associated with functional capacity. We propose that simultaneous evaluation of biventricular function should provide incremental predictive value for exercise intolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nonobstructive hypertrophic cardiomyopathy (NHCM) is a relatively common (approximately 33%) [1] inherited cardiomyopathy caused by dominant mutations in genes encoding sarcomere-related proteins. NHCM is a type of hypertrophic cardiomyopathy (HCM) that is characterized by a left ventricular outflow tract gradient < 30 mmHg both at rest and during exercise [2].
The clinical manifestations of NHCM are heterogeneous, due to left ventricular (LV) or biventricular hypertrophy, chronotropic incompetence, myofiber disarray or interstitial fibrosis, and microvascular ischemia [3, 4], and can present with either no-symptoms or result in sudden cardiac death [5]. Reduced exercise capacity due to LV or biventricular diastolic dysfunction is a common symptom among NHCM patients. Furthermore, several studies have demonstrated that exercise intolerance is a strong predictor of adverse cardiovascular events in NHCM patients [6, 7]. A substantial proportion of NHCM patients are at a low risk of advanced heart failure and HCM-related mortality, as has been supported by current expert reviews [8, 9]. However, recent accumulating evidence has demonstrated that NHCM patients have a more severe burden of fibrosis and higher rates of microvascular ischemia and ventricular arrhythmias [10, 11]. Moreover, the long-term mortality of NHCM patients was comparable to that of obstructive HCM patients [12]. In addition, the characterized histological hallmark of HCM patients is not confined to the left ventricle; thus, the right ventricle may also be involved in NHCM and manifest dysfunction, which may also contribute to reduced exercise capacity [13]. Numerous previous studies have mainly focused on abnormal LV function, but only a few studies have simultaneously evaluated biventricular mechanics in NHCM patients. Hence, the respective contribution of LV and right ventricular (RV) function to exercise capacity, particularly the value of biventricular mechanics for predicting exercise intolerance in NHCM patients, remains unclear.
Exercise echocardiography (EE) is typically used to evaluate exercise capacity in HCM patients, but can also provide incremental prognostic information and risk stratification [14]. Accordingly, EE has been strongly recommended to assess cardiac function of HCM patients according to the current guidelines [15]. Two-dimensional speckle-tracking imaging (2D-STI) has also gained increasing utility for diagnosing HCM patients owing to earlier detection of subclinical myocardial dysfunction [16, 17]. However, there are limited studies on the integration of EE and 2D-STI for determining biventricular mechanics in NHCM patients.
The present study aimed to assess changes in biventricular mechanics at rest and during exercise and to explore the association between exercise capacity and biventricular mechanics and functional reserve in NHCM patients. We also examined whether simultaneous evaluation of biventricular function could provide incremental predictive information for exercise intolerance.
Methods
Study population
This study included 67 NHCM patients who were consecutively recruited in the Department of Echocardiography, Heart Center, Beijing Chao Yang Hospital between June 2015 and July 2018. The inclusion criteria were based on current published guidelines: maximal LV wall thickness ≥ 15 mm or between 13 and 15 mm, positive family history, or abnormal electrocardiography without another explainable cause that may produce a similar magnitude of LV hypertrophy [18]. NHCM was defined as having an LV outflow tract gradient (LVOTG) < 30 mmHg both at rest and during exercise. Exclusion criteria included: history of coronary artery disease, hypertension, diabetes mellitus, heart failure with New York Heart Association (NYHA) functional classes III–IV, history of ventricular septal reduction therapy, severe valvular disease or valvular prostheses, congenital heart disease, and poor imaging quality. Patients with percutaneous coronary intervention (n = 3), liable obstructive HCM (n = 6), and poor image quality (n = 8) were also excluded. After application of the inclusion and exclusion criteria, 50 NHCM patients were enrolled in this study (Supplemental Fig. 1). In addition, 25 age-and-sex-matched healthy subjects were registered as controls. This was a case-control study that was approved by the local ethics committee. Written informed consent was obtained from all participants.
Echocardiography
Conventional measurements
Cardiac functions of all participants were examined at rest and during exercise, and standardized views were acquired with the subject in the left lateral decubitus position using a commercially available ultrasound machine (EPIQ 7C, Philips Healthcare, MA, USA) equipped with an X5-1 multiphase-array probe. Conventional measurements were performed in line with the current recommendations of the American Society of Echocardiography [19]. LV dimensions were determined at the parasternal long-axis view, and LV ejection fraction (LVEF) was calculated using the modified biplane Simpson’s method. Peak early (E) and late (A) wave velocities of mitral and tricuspid filling were recorded at the level of corresponding tips. Mitral annular peak velocities at the septal and lateral levels during early diastole (e′) were determined using pulsed tissue Doppler, and the mean value of the septal and lateral e′ was calculated. The E/e′ parameter was also evaluated, which was an indicator of LV filling pressure. Tricuspid annular peak velocities were measured by M-mode. LVOTG was estimated using the simplified Bernoulli Eq. \(\left[ {4\, \times \,{{\left( {{\text{LVOT peak velocity}}} \right)}^2}} \right]\). LV mass was measured through three-dimensional echocardiography, and the calculated LV mass was indexed to body surface area to calculate LV mass index (LVMI). RV free wall thickness (RVWT) was measured at the subcostal view.
Deformation analysis
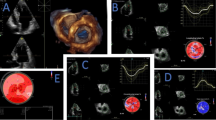
Two-dimensional grayscale images were collected at rest and during exercise by recording three consecutive heart cycles, with the frame rate optimized to 50–100 frames/s. 2D-STI offline analyses were performed with the dedicated software QLAB 10.3 software (Philips Healthcare, USA), which can automatically track the endocardial contour, and the region of interest was adjusted manually if necessary. If the entire LV wall or RV free wall was not included in the region of interest, it was further adjusted manually for optimization. To guarantee that all segments were tracked appropriately, the pericardium was excluded when a strain analysis was performed. Peak LV global longitudinal strain (LVGLS) (Fig. 1A, B) and strain rate (SR) of each phase were measured from the LV apical two, three, and four-chamber views, and peak LV global circumferential strain (LVGCS) was determined at the parasternal basal, middle, and apical short-axis views. Peak RV free wall longitudinal strain (RVFWLS) was obtained from three segments of the RV free wall (Fig. 1C, D). The reserve of LV and RV strain or SR was calculated as the difference between peak exercise and rest values.
Evaluation of biventricular mechanics in a patient with NHCM using two-dimensional speckle-tracking imaging. LV longitudinal strain was determined in an apical four-chamber view at rest (A) and peak exercise (B). RV free wall longitudinal strain was measured in an RV-focused four-chamber view at rest (C) and peak exercise (D)
Exercise protocol
Multistage, symptom-limited, semi-supine exercise testing was implemented by a bicycle ergometer (Ergoselect 1200, Stress Echo Couch Ergometer; Ergoline, Bitz, Germany) after the resting echocardiography. Workload began at 25 W, with subsequent stepwise increases of 25 W every 2 min. Participants were encouraged to exercise until exhaustion with a rate of 55–65 rounds/min. Heart rhythm, heart rate, and blood pressure were continuously monitored. Exercise testing was promptly interrupted in cases of one or more of the following: age-related maximum heart rate (HR), significant ventricular arrhythmia, severe hypertension (blood pressure ≥ 240/120 mmHg), muscle fatigue, or symptom intolerance such as severe breathlessness, chest pain, and dizziness. β-blockers or calcium channel blockers were stopped at least 24 h before exercise tests. The maximal HR, peak systolic blood pressure (SBP), metabolic equivalents (METs), and peak rate–pressure product (RPP = maximal HR × SBP) were recorded at peak exercise. Patients with reduced exercise capacity were defined as having METs < 7 [20].
Statistical analysis
Quantitative variables are expressed as mean ± standard deviation (SD), and categorical variables are presented as absolute values and percentages. Normal distribution was assessed with the Kolmogorov–Smirnov test. The t test and the Mann–Whitney U test were used to compare continuous variables for normally and non-normally distributed data, respectively, while the χ2 was performed to compare categorical data. Pearson’s or Spearman’s test was used to determine correlations between exercise capacity and other parameters. Multivariable regression analysis was performed to evaluate independent predictors of the reduced exercise capacity in NHCM patients. Receiver operating characteristic (ROC) curves were introduced to identify the sensitivity and specificity of parameters for predicting exercise intolerance. Global χ2 analysis was adopted to evaluate whether simultaneous evaluation of biventricular function could provide the incremental predictive value for exercise intolerance over the assessment of individual ventricular function alone. Inter- and intra-observer agreement of measurements was evaluated by intraclass correlation coefficients (ICC) and the coefficient of variation (CV). Statistical analyses were performed using SPSS 23.0 (IBM SPSS Statistics, version 23, USA) and MedCalc 15.6 (MedCalc Software, Ostend, Belgium), and a two-tailed P value < 0.05 indicated a statistically significant difference.
Results
Demographic and baseline clinical characteristics
A total of 75 individuals in a study population were finally recruited with the demographic and baseline clinical characteristics at rest as presented in Table 1. Among this study population, 50 were NHCM patients (mean age of 52.9 ± 11.4 years) and 25 were healthy controls (mean age of 49.2 ± 10.1 years). There was no difference in gender between these two groups. Among the 50 NHCM patients, 88% NHCM patients were male, 16% NHCM patients received calcium antagonists, and 58% NHCM patients used β-blockers. Additionally, NHCM patients had significantly higher LVE/e′ (LV filling pressure) and absolute values of LVGCS and lower absolute values of LVGLS at rest compared to the control subjects. Age, body surface area, and LVEF were comparable between the two groups. Although there were no significant differences in RV fractional area change, tricuspid annular plane systolic excursion, and the tricuspid peak E/A ratio between NHCM patients and controls, the absolute values of RVFWLS were significantly lower in NHCM patients compared to the control subjects.
Biventricular mechanics and reserve at peak exercise
LV and RV mechanics and functional reserve at peak exercise are summarized in Table 2. NHCM patients had higher LV filling pressure, as demonstrated by a higher LVE/e′-exe ratio. NHCM patients also had significantly impaired LV systolic and diastolic mechanics at peak exercise, as evidenced by reduced absolute values of LVGLS, LVGCS, LV systolic strain rate (LVSRs), and lower early diastolic strain rate (LVSRe). Additionally, LV systolic and diastolic reserve were significantly lower in NHCM patients compare to controls, as evidenced by a lower ΔLVGLS (− 3.64 ± 1.48% vs − 4.88 ± 2.19%, P = 0.018) and ΔLVSRe (0.25 ± 0.14 vs 0.42 ± 0.13, P < 0.001). Similarly, RVFWLS and RV systolic reserve were also significantly lower in NHCM patients compared to controls.
Hemodynamics at peak exercise
Hemodynamics of the study population at peak exercise are presented in Table 3. NHCM patients had a significantly lower achieved maximal HR and peak RPP compared to controls. Moreover, NHCM patients exhibited lower METs compared to control subjects. No death or syncope events occurred during exercise tests in both groups.
Correlations between exercise capacity and biventricular function
Table 4 and Fig. 2 summarize the correlations between exercise capacity and biventricular function in NHCM patients. METs were negatively correlated with LVGLS-exe, LVGCS-exe, LVE/e′-exe, and RVFWLS-exe (r = − 0.62, r = − 0.37, r = − 0.63, and r = − 0.49, respectively; P < 0.01 for all). Multivariable logistic regression showed that LVE/e′-exe (odds ratio (OR): 1.64; 95% confidence interval (CI) 1.20 to 2.24; p = 0.002) and RVFWLS-exe (OR 1.65; 95% CI 1.09 to 1.51; p = 0.019) were independent predictors of reduced exercise capacity in NHCM patients.
Predictors of exercise intolerance in NHCM patients
As demonstrated in Fig. 3, ROC analysis showed the ability of LVGLS-exe, LVE/e′-exe, and RVFWLS-exe to predict exercise intolerance in NHCM patients based on METs. LVGLS-exe ≥ − 23.3% predicted exercise intolerance (METs < 7) with an area under the curve (AUC) of 0.754, a sensitivity of 89.7%, and a specificity of 52.4%; LVE/e′-exe ≥ 15.1 predicted exercise intolerance with an AUC of 0.883, a sensitivity of 72.4%, and a specificity of 95.2%; and RVFWLS-exe ≥ − 20.3% predicted exercise intolerance with an AUC of 0.830, a sensitivity of 69.0%, and a specificity of 90.5%. Figure 4 shows the associations between biventricular function and exercise intolerance assessed using the global χ2 test. Sequential addition of LVE/e′-exe and RVFWLS-exe to the clinical model of LVGLS-exe provided an incremental predictive value for reduced exercise capacity in NHCM patients (Fig. 4A). Similar increments in global Chi square were noted when RVFWLS-exe was evaluated after LVGLS-exe followed by LVE/e′-exe for exercise intolerance in NHCM subjects (Fig. 4B).
Inter- and intra-observer reproducibility
Fifteen study subjects (10 NHCM patients and 5 controls) were randomly selected from each group and images were measured by the original investigator and a second experienced observer one month after the initial evaluation. Both observers were blinded to each other’s analysis. ICCs of inter- and intra-observer variability were 0.81 and 0.84 for LVGLS-exe, and 0.80 and 0.85 for RVFWLS-exe, respectively. The CVs for inter- and intra-observer variability were − 5.5% and − 5.0% for LVGLS-exe, and − 5.9% and − 5.4% for RVFWLS-exe, respectively.
Discussion
The present study provided several new insights into the association between biventricular function and exercise performance in NHCM patients: (1) NHCM patients had lower biventricular longitudinal mechanics and higher LV circumferential systolic function at rest, and exhibited significantly impaired biventricular mechanics and functional reserve; (2) biventricular function correlated with exercise capacity in NHCM patients; (3) in NHCM patients, LVE/e′-exe and RVFWLS-exe were independent predictors of reduced exercise performance; and (4) simultaneous evaluation of biventricular function may provide an incremental predictive value over individual LV or RV function alone for reduced exercise capacity in NHCM patients.
Impaired LV mechanics in NHCM patients
The NHCM population accounts for approximately for one-third of all HCM patients [21]. While a more recent meta-analysis showed that the long-term mortality of NHCM patients was comparable to that of obstructive HCM patients [12], NHCM patients usually have LV dysfunction, which is an important cause of major adverse cardiovascular events, such as heart failure or sudden cardiac death [8, 22]. Consistent with the above observations, in this study, we found that NHCM patients had significantly lower absolute LVGLS values and higher LVGCS values at rest compared to controls, but both were impaired during exercise. Thus, the present study validated and extended the findings of Carasso et al. who illustrated that LVGCS was significantly enhanced at rest in HCM patients [23]. Our results further support that LVGLS is more sensitive than LVEF for identifying subclinical myocardial systolic dysfunction in NHCM patients, as demonstrated by Moneghetti et al. [24]. We propose that enhanced LVGCS observed at rest in NHCM patients compensates for a reduction in LVGLS.
Accumulating evidence has shown that LV diastolic dysfunction is an important determinant of reduced exercise capacity and adverse outcomes in HCM patients [25]. In line with the previous findings, we also found that NHCM patients had impaired LV diastolic function, which manifested as a lower LV SRe and a higher LVE/e′ compared to controls both at rest and during exercise. The LVE/e′ is important for assessing LV filling pressure in HCM patients and has been recommended for use in clinical practice by current guidelines [26]. Also, it has recently been reported that LV diastolic reserve evaluated by ΔLVE/e′ was strongly associated with exercise capacity, and that LVE/e′ at rest is an independent predictor of cardiovascular events, such as HCM-related death, new onset of atrial fibrillation, and worsening of heart failure [25, 27]. Thus, LVE/e′ may be used for risk stratification and prediction of the prognosis of NHCM patients. Given the interaction between LV systolic and diastolic functions, it is conceivable that the coexistence of systolic and diastolic dysfunction may impair LV systolic-diastolic coupling efficiency. This impaired coupling efficiency will eventually cause ineffective augmentation of LV stroke volume during exercise, according to Frank-Starling’s law, leading to reduced exercise capacity in NHCM patients.
Impaired LV mechanics are ascribed to several pathophysiological mechanisms in NHCM patients. In terms of coronary microcirculation, myocardial ischemia is attributed to remodeling of intramural arterioles, which results in LV dysfunction [3]. It was reported that LVGLS was mainly determined by subendocardial fibers, which are more sensitive to abnormal myocardial perfusion. Therefore, consistent with a previous study by Okada et al. [28], we found that LVGLS was reduced in NHCM patients in the current study. In contrast, abnormal histological structures, such as myocyte disarray, replacement fibrosis, and interstitial fibrosis, is mainly due to gene mutations and further undermines LV systolic and diastolic function. Indeed, a recent study by Nucifora et al. showed that the extent of replacement fibrosis was negatively correlated with LV systolic function, while the extent of interstitial fibrosis mainly affected LV diastolic function in HCM patients [29].
Abnormal RV systolic function in NHCM patients
Given that LV hypertrophy is mainly associated with LV dysfunction in HCM patients, it is understandable that many previous HCM studies have primarily concentrated on LV mechanics and LVOTG, which largely determine clinical management strategies [14, 22]. As such, these studies omitted the right ventricle, which may also be dysfunctional and potentially impact the prognosis of HCM patients. In the present study, we showed that NHCM had higher RV free wall thickness compared to controls, suggesting that the myocardial remodeling that occurred in NHCM patients was not limited to LV. This finding was in accordance with other several previous studies [30, 31]. Additionally, we also observed that the indices of RIMP and tricuspid E/e′ were higher in NHCM patients compared to control subjects, indicating that the RV filling pressure was increased in NHCM patients, which further corroborated the previous observation by Pagourelias et al. [32] that HCM patients had RV diastolic dysfunction that served as an independent predictor of poor HCM prognosis. Our study further revealed that RV systolic dysfunction was present in NHCM patients, as evidenced by lower RVFWLS at rest and during exercise, as previously reported [33]. Therefore, we provide evidence that abnormal RV mechanics in NHCM patients should also be regarded as an important factor for risk stratification.
Currently, the precise mechanisms leading to RV dysfunction in NHCM patients are unclear. Anatomically, abnormal RV mechanics may be associated with the hypertrophied interventricular septum shared by the LV and RV. Guo et al. compared the histopathological changes between LV and RV myocardial specimens, and found no difference in microscopic histological features [34], implying that an extension of the LV myopathic process may underlie impaired RV mechanics. Alternatively, sarcomere dysfunction caused by gene mutations could contribute to reduced RV mechanics [35].
Impact of biventricular function on functional capacity
It is well acknowledged that LV or RV dysfunction could result in reduced exercise capacity in HCM patients, and eventually progress to heart failure or sudden cardiac death. Therefore, increasing evidence supports the notion that exercise intolerance may serve as an independent risk factor of major adverse cardiovascular events in NHCM patients [7, 20]. In the present study, we found that NHCM patients had reduced exercise capacity determined by METs, which correlated with LVGLS at rest and during exercise. Our findings further emphasize the importance of evaluating LV systolic function. Moreover, our study revealed that higher LVE/e′-exe is an independent predictor of reduced exercise performance, indicating that worsening diastolic function could result in reduced cardiac output and exacerbate clinical symptoms of NHCM patients. Furthermore, our study also showed that evaluating RV systolic dysfunction determined by RVFWLS improved the prediction of exercise intolerance beyond LV systolic and diastolic dysfunction in NHCM patients and vice versa. Hence, we argue that simultaneous evaluation of biventricular function may contribute to improved risk stratification of NHCM patients in clinical practice.
Clinical implications
To the best of our knowledge, this is the first study to simultaneously evaluate LV and RV mechanics at rest and during exercise in NHCM patients. We found that NHCM patients had both LV and RV dysfunction, and that concurrent assessment of biventricular mechanics during exercise presented an incremental predictive value of impaired exercise capacity, which is correlated with major adverse cardiovascular events. Although increasing evidence suggests that NHCM patients have a higher risk of suffering from malignant arrhythmia events, worsening heart failure, and death [10, 11, 36], the current predictive model of sudden cardiac death cannot effectively identify high risk patients. Thus, this study is expected to offer a new means for improved risk stratification of NHCM patients.
Limitations
Several limitations of this study should be acknowledged. First, this study was performed in a single tertiary center with a relatively small sample size; thus, the results may be confounded by inherent selection bias. Second, given that the subjects of this study only included NHCM patients, we can not necessarily extrapolate our findings to obstructive HCM patients. Third, since we did not have access to commercial software specialized for RV strain imaging, RVFWLS was evaluated using LV dedicated software. Fourth, although the imaging gold standard is cardiac magnetic resonance, we did not utilize this approach due to its being time-consuming and costly. Echocardiography is the most common method of examination for HCM patients in clinical practice, which can be used to effectively evaluate cardiac structure and function in NHCM patients and is recommended by current guidelines. Although image quality is susceptible to respiratory motion, echocardiography is an inexpensive, convenient, and point-of-care imaging tool. Finally, the incremental prognostic value of simultaneous evaluation of biventricular function should be confirmed by prospective large cohort studies in the future.
Conclusions
Biventricular longitudinal mechanics and functional reserve during exercise in NHCM patients are markedly impaired. Biventricular function is also closely associated with exercise performance in NHCM patients, and thus simultaneous evaluation of biventricular function may provide an incremental predictive value for exercise intolerance. These findings suggest that exercise strain parameters of biventricular function may be used to predict prognosis of NHCM patients. However, our proposed clinical application of biventricular function in NHCM patients requires corroboration in multicenter prospective studies in the future.
References
Gersh BJ, Maron BJ, Bonow RO et al (2011) 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 124:2761–2796
Maron BJ, Maron MS (2013) Hypertrophic cardiomyopathy. Lancet 381:242–255
Tower-Rader A, Betancor J, Lever HM, Desai MY (2017) A comprehensive review of stress testing in hypertrophic cardiomyopathy: assessment of functional capacity, identification of prognostic indicators, and detection of coronary artery disease. J Am Soc Echocardiogr 30:829–844
Efthimiadis GK, Giannakoulas G, Parcharidou DG et al (2011) Chronotropic incompetence and its relation to exercise intolerance in hypertrophic cardiomyopathy. Int J Cardiol 153:179–184
Geske JB, Ommen SR, Gersh BJ (2018) Hypertrophic cardiomyopathy: clinical update. JACC Heart Fail 6:364–375
Peteiro J, Bouzas-Mosquera A, Fernandez X, Monserrat L, Pazos P, Estevez-Loureiro R, Castro-Beiras A (2012) Prognostic value of exercise echocardiography in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr 25:182–189
Desai MY, Bhonsale A, Patel P, Naji P, Smedira NG, Thamilarasan M, Lytle BW, Lever HM (2014) Exercise echocardiography in asymptomatic HCM: exercise capacity, and not LV outflow tract gradient predicts long-term outcomes. JACC Cardiovasc Imaging 7:26–36
Maron MS, Rowin EJ, Olivotto I et al (2016) Contemporary natural history and management of nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 67:1399–1409
Maron BJ, Rowin EJ, Maron MS, Braunwald E (2017) Nonobstructive hypertrophic cardiomyopathy out of the shadows: known from the beginning but largely ignored … until now. Am J Med 130:119–123
Pozios I, Corona-Villalobos C, Sorensen LL et al (2015) Comparison of outcomes in patients with nonobstructive, labile-obstructive, and chronically obstructive hypertrophic cardiomyopathy. Am J Cardiol 116:938–944
Pozios I, Pinheiro A, Corona-Villalobos C et al (2018) Rest and stress longitudinal systolic left ventricular mechanics in hypertrophic cardiomyopathy: implications for prognostication. J Am Soc Echocardiogr 31:578–586
Pelliccia F, Pasceri V, Limongelli G et al (2017) Long-term outcome of nonobstructive versus obstructive hypertrophic cardiomyopathy: a systematic review and meta-analysis. Int J Cardiol 243:379–384
D’Andrea A, Limongelli G, Baldini L et al (2017) Exercise speckle-tracking strain imaging demonstrates impaired right ventricular contractile reserve in hypertrophic cardiomyopathy. Int J Cardiol 227:209–216
Rowin EJ, Maron BJ, Olivotto I, Maron MS (2017) Role of exercise testing in hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 10:1374–1386
Lancellotti P, Pellikka PA, Budts W et al (2017) The clinical use of stress echocardiography in non-ischaemic heart disease: recommendations from the European association of cardiovascular imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 30:101–138
Tower-Rader A, Mohananey D, To A, Lever HM, Popovic ZB, Desai MY (2018) Prognostic value of global longitudinal strain in hypertrophic cardiomyopathy: a systematic review of existing literature. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2018.07.016
Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S (2016) Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J 37:1196–1207
Authors/Task Force m, Elliott PM, Anastasakis A et al (2014) 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the european society of cardiology (ESC). Eur Heart J 35:2733–2779
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–270
Badran HM, Faheem N, Ibrahim WA, Elnoamany MF, Elsedi M, Yacoub M (2013) Systolic function reserve using two-dimensional strain imaging in hypertrophic cardiomyopathy: comparison with essential hypertension. J Am Soc Echocardiogr 26:1397–1406
Gersh BJ, Maron BJ, Bonow RO et al (2011) 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 58:e212–e260
Maron BJ, Rowin EJ, Udelson JE, Maron MS (2018) Clinical spectrum and management of heart failure in hypertrophic cardiomyopathy. JACC Heart Fail 6:353–363
Carasso S, Yang H, Woo A, Vannan MA, Jamorski M, Wigle ED, Rakowski H (2008) Systolic myocardial mechanics in hypertrophic cardiomyopathy: novel concepts and implications for clinical status. J Am Soc Echocardiogr 21:675–683
Moneghetti KJ, Stolfo D, Christle JW, Kobayashi Y, Finocchiaro G, Sinagra G, Myers J, Ashley EA, Haddad F, Wheeler MT (2017) Value of strain imaging and maximal oxygen consumption in patients with hypertrophic cardiomyopathy. Am J Cardiol 120:1203–1208
Choi EY, Ha JW, Rim SJ, Kim SA, Yoon SJ, Shim CY, Kim JM, Jang Y, Chung N, Cho SY (2008) Incremental value of left ventricular diastolic function reserve index for predicting exercise capacity in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr 21:487–492
Nagueh SF, Smiseth OA, Appleton CP et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr 29:277–314
Kitaoka H, Kubo T, Hayashi K, Yamasaki N, Matsumura Y, Furuno T, Doi YL (2013) Tissue Doppler imaging and prognosis in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging 14:544–549
Okada K, Yamada S, Iwano H et al (2015) Myocardial shortening in 3 orthogonal directions and its transmural variation in patients with nonobstructive hypertrophic cardiomyopathy. Circ J 79:2471–2479
Nucifora G, Muser D, Gianfagna P, Morocutti G, Proclemer A (2015) Systolic and diastolic myocardial mechanics in hypertrophic cardiomyopathy and their link to the extent of hypertrophy, replacement fibrosis and interstitial fibrosis. Int J Cardiovasc Imaging 31:1603–1610
Cheng TO (2008) Hypertrophic cardiomyopathy is a biventricular disease. Int J Cardiol 129:3–4
Rosca M, Calin A, Beladan CC et al (2015) Right ventricular remodeling, its correlates, and its clinical impact in hypertrophic cardiomyopathy. J Am Soc Echocardiogr 28:1329–1338
Pagourelias ED, Efthimiadis GK, Parcharidou DG, Gossios TD, Kamperidis V, Karoulas T, Karvounis H, Styliadis IH (2011) Prognostic value of right ventricular diastolic function indices in hypertrophic cardiomyopathy. Eur J Echocardiogr 12:809–817
Afonso L, Briasoulis A, Mahajan N, Kondur A, Siddiqui F, Siddiqui S, Alesh I, Cardozo S, Kottam A (2015) Comparison of right ventricular contractile abnormalities in hypertrophic cardiomyopathy versus hypertensive heart disease using two dimensional strain imaging: a cross-sectional study. Int J Cardiovasc Imaging 31:1503–1509
Guo X, Fan C, Wang H, Zhao S, Duan F, Wang Z, Yan L, Yang Y, An S, Li Y (2016) The prevalence and long-term outcomes of extreme right versus extreme left ventricular hypertrophic cardiomyopathy. Cardiology 133:35–43
Marian AJ, Braunwald E (2017) Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res 121:749–770
Lu DY, Pozios I, Haileselassie B, Ventoulis I, Liu H, Sorensen LL, Canepa M, Phillip S, Abraham MR, Abraham TP (2018) Clinical outcomes in patients with nonobstructive, labile, and obstructive hypertrophic cardiomyopathy. J Am Heart Assoc 7:e006657
Acknowledgements
We are very grateful to all subjects for their participation and other colleagues for their support.
Funding
The present study was supported by the National Natural Science Foundation of China (NSFC No. 81571683), Beijing Chao-yang Hospital 1351 Talent Training Plan (No. CYMY-2017-28).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the local ethics committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
. Flowchart showing the enrollment of subjects in the study. PCI = percutaneous coronary intervention (TIF 192 KB)
Rights and permissions
About this article
Cite this article
Wu, XP., Li, YD., Wang, YD. et al. Decreased biventricular mechanics and functional reserve in nonobstructive hypertrophic cardiomyopathy patients: implications for exercise capacity. Int J Cardiovasc Imaging 35, 869–879 (2019). https://doi.org/10.1007/s10554-019-01530-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-019-01530-y