Abstract
Pulmonary arteriovenous malformation, a condition most commonly associated with hereditary hemorrhagic telangiectasia, is an abnormal communication between the pulmonary artery and pulmonary vein without an intervening capillary communication. Although asymptomatic in ~ 50% individuals, it can present with the dreaded complications of stroke or intracranial abscess in high-risk individuals including pregnant women, if untreated. The mainstay of treatment is now endovascular embolization of the feeding artery which can alleviate the symptoms and prevent these complications. In this review, we describe the pathophysiology, methods of screening, diagnostic workup and treatment of these vascular lesions with a particular focus on the currently used embolization techniques and their outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary arteriovenous malformation (PAVM) is an abnormal communication between the pulmonary artery and pulmonary vein without an intervening capillary communication. It may be associated with a venous aneurysm (sac) at the site of the communication. PAVM was initially described by Churton in the nineteenth century, like a walnut-sized lesion filled with a blood clot in a postmortem child [1]. PAVMs have also been addressed as pulmonary arteriovenous fistulae, pulmonary arteriovenous aneurysms, cavernous angiomas of the lung and pulmonary telangiectasias. PAVMs are usually asymptomatic, and the majority is detected incidentally. When large or multiple, however, they may become symptomatic due to the right-to-left shunt effect. Life-threatening complications of PAVM include stroke, transient ischemic attack, cerebral abscess, massive hemoptysis, and spontaneous hemothorax [2,3,4]. Prompt treatment, therefore, is essential to minimize patients’ morbidity and mortality.
Epidemiology
PAVMs were first associated with Hereditary Hemorrhagic Telangiectasia (HHT) in 1938 [5]. HHT, also known as Osler–Weber–Rendu syndrome, is an autosomal dominant disorder prevalent in more than 1 in 10,000 individuals [6,7,8]. HHT is characterized by a triad of epistaxis, mucocutaneous or visceral telangiectasia and family history of PAVM. Endoglin (ENG) gene on chromosome 9 (HHT1 phenotype), activin receptor-like kinase-1 ACVRLI/ALK1 gene on chromosome 12 (HHT2 phenotype) and SMAD4 gene on chromosome 18 have been linked to the inheritance of the disease [9]. Around 15–50% of patients with HHT have PAVM [2, 10, 11]. Around 70% of PAVM are a part of HHT syndrome, and the rest are sporadic [2, 12]. PAVMs are more common and have a larger shunt when associated with ENG mutation (58%) versus ACRVL1 mutation (18%) on chromosome 9 [13]. The estimated prevalence of PAVMs is 38/100,000 individuals [14]. PAVMs are known to have a female preponderance [14, 15], and with pregnancy being a risk factor for complications from PAVMs, it is important to screen high-risk individuals [10, 16]. Acquired PAVM occur in individuals with prior cavopulmonary shunt procedures for congenital heart diseases, chronic liver disease or history of tuberculosis or actinomycosis infections [17, 18].
Pathophysiology
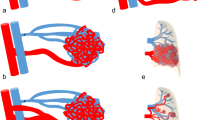
Mutations of the ENG and ACRVL1 alter the ligand-receptor interactions at the endothelial surface while the SMAD4 mutation interferes with the intracellular signaling within the endothelium. The imbalance between the proangiogenic vascular endothelial growth factor (VEGF) and the antiangiogenic transforming growth factor-β (TGF-β) leads to the formation of persistent thin walled direct arteriovenous conduits exposed to arterial blood flow and increased shear forces [19]. In rare cases, the fragile walls of PAVMs may rupture and cause hemothorax and hemoptysis. 65% of PAVMs favor lower lobes probably due to the increased pulmonary blood flow and pressure which is the cause of platypnea, i.e., dyspnea relieved on lying flat and orthodeoxia, i.e., desaturation in an upright position [20]. Around 90% of PAVMs have a single feeding artery and are characterized as “simple” [20, 21] (Fig. 1). Five percent are “complex” PAVMs with two or more feeding arteries from different segments. Another five percent are “diffuse” PAVMs with many feeders [22, 23].
Clinical features
Approximately 13–56% affected individuals with PAVMs are asymptomatic [24]. A careful physical examination may detect 75% of cases in the high-risk populations [25]. The presence of symptoms best correlates with the size of the PAVMs rather than the number of the lesion [24]. The presenting symptoms may include dyspnea, intrapulmonic hemorrhage, neurological symptoms/deficits, palpitations, cough, and chest pain. Signs which may be present on physical examination may include bruit/thrill, clubbing, telangiectasia, polycythemia, cyanosis or a systolic murmur. Complications associated with PAVMs typically arise from two mechanisms. The first results from the decreased O2 partial pressure and oxygen saturation in the systemic arterial supply. The sequela of this abnormal phenomenon can lead to hypoxemia, anemia, hemoptysis, hemothorax, and pulmonary hypertension. The second mechanism involves anomalous venous drainage. This abnormal arterio-venous communication can lead to pulmonary artery hypertension, but more commonly, complications from paradoxical embolization such as transient ischemic accidents or stroke, cerebral abscesses, endocarditis, or visceral or extremity ischemia/infarction.
Screening
Physical examination including thorough ear, nose and throat examination, chest radiography, arterial blood gas measurements, and finger oximetry are essential in screening individuals with suspected PAVMs [26]. Transthoracic contrast echocardiography (TTCE) is the recommended initial screening test for PAVM detection in high-risk patients [27]. The principal behind TTCE in the detection of shunts is dependent on the signal produced by agitated saline which produces numerous microbubbles. Newer ultrasound contrast agents developed recently have facilitated increased duration of the contrast material in the blood pool, i.e., Lumason (Bracco Diagnostics). TTCE is considered positive if there is the detection of any bubbles in the left atrium. Around 44–60% of individuals with HHT may have positive contrast echocardiography indicative of an intrapulmonary shunt [28, 29]. Positive screening can be confirmed with non-contrast multidetector thoracic CT with thin-slice (e.g. 1–2 mm) reconstructions. In children, screening tests may include a physical exam (for cyanosis, dyspnea, clubbing), supine and upright pulse oximetry, chest radiography and/or TTCE. A combination of a chest radiograph and contrast echocardiography achieves nearly 100% sensitivity and negative predictive value [11, 30]. In high-risk patients with negative initial screening, repeat screening should be considered after puberty, after pregnancy, within 5 years preceding planned pregnancy and otherwise every 5–10 years [27]. With the advancement of genetic mutation testing and family mapping, 80% of affected individuals can be identified by testing for the three most common mutations [31].
Diagnosis and work-up
Patients with HHT are diagnosed based on the Curaçao Criteria [32] (Table 1). Comprehensive blood evaluation, electrocardiography, CT pulmonary angiography, contrast-enhanced echocardiography or radionuclide angiocardiography (to avoid angiography in nonsurgical cases and atypical cases) are the necessary investigations [24]. Optional investigations include catheter-directed angiography, perfusion lung scan, cardiac catheterization, and shunt evaluation procedures.
On chest radiograph, PAVMs appear as homogeneous sharply demarcated pulmonary lesions, with associated curvilinear opacities demonstrating the feeding artery and a draining vein coursing towards the hilum [25, 33]. PAVMs may also appear as a more complex plexiform mass of dilated vascular channels or as dilated tortuous direct communication between an artery and vein [34].
TTCE is minimally invasive initial screening modality with high sensitivity for detecting shunts in the lungs. A grading system (from 0 to 3) has been proposed and studied as a screening tool for PAVMs in HHT patients. The grade is determined by the appearance and concentration of bubble contrast in the left atrium after a minimum of three cardiac cycles, wherein, Grade 0, no bubbles; Grade 1, occasional filling with < 20 bubbles; Grade 2, moderate filling; Grade 3, complete opacification. The overall diagnostic performance was found to have a sensitivity of 1.00, specificity of 0.49, positive predictive value (PPV) of 0.32 and negative predictive value (NPV) of 1.00 [35].
CT pulmonary angiography is considered the gold standard for diagnosis of PAVMs. At our institution, PAVM surveillance and follow-up are performed with CT angiograms following a pulmonary embolism protocol. We recommend a helical scan using 0.63 mm collimation with 1 mm and 3 mm axial reconstructions. We image after injecting 90 cm3 of contrast at a rate of 5 cm3/s with bolus tracking over the main pulmonary artery. 2 mm coronal and sagittal multiplanar reformatted (MPR) images, as well as 5 × 5 and 7 × 2 coronal and axial maximum intensity projections (MIPS), are also submitted. In particular, the MIP images provide a more sensitive means to detect smaller PAVMs. Employing 3D reconstruction software can depict the angioarchitecture of the feeding arteries and draining veins (number, size, and orientation of the vessels) which can serve as a roadmap for selective embolotherapy and also helps estimate the size of the PAVM before and after embolotherapy [36, 37]. Given the possibility of multiple and bilateral PAVMs, imaging with CT is extremely valuable to both assess the extent of the condition as well as to plan endovascular treatment. Because of this, emergent embolotherapy without a CT is exceedingly rare.
Magnetic Resonance Imaging (MRI) has seen greater usage in detecting, pre-embolization planning and evaluating treated PAVMs. MRI, compared to CT, has the benefit of avoiding ionizing radiation as well as the use of iodinated contrast, particularly in a patient with allergies or renal insufficiency [38, 39]. A study comparing the detection rates of contrast-enhanced MR angiography (CE-MRA) with conventional pulmonary angiography concluded that CE-MRA could detect PAVMs as small as 2 mm and can potentially be used as a screening tool for detecting PAVMs [40]. A suggested protocol for performing dynamic contrast-enhanced MR angiogram includes a three-dimensional T1-weighted spoiled gradient echo sequence after intravenous injection of 0.1–0.2 mmol/kg gadolinium at a flow rate of 2–5 cm3/s to shorten T1. Scan acquisition commences at the peak of the contrast enhancement by centric elliptic phase encoding and can be completed in two breath holds [41].
Management of PAVM
Before 1978, surgery was the only option to treat PAVMs, and this entailed local excision, segmental resection, lobectomy, or pneumonectomy to reduce complications in symptomatic patients [24]. Initial reports of embolotherapy were published in 1978 [42] and 1980 [43]. However, the International Guidelines by an expert panel now agree that surgical management of PAVMs plays a minimal role, other than in the management of life-threatening bleeding in a center where there is no expertise in embolotherapy [27].
“Single-session outpatient embolotherapy” is the ideal approach to managing PAVM patients with four or fewer lesions [44]. Patients with multiple lesions may require multiple sessions of outpatient therapy (Fig. 2). Treatment of patients with PAVMs is recommended for both symptomatic adults and children and asymptomatic adults. The decision to treat asymptomatic children should occur on a case-by-case basis, and embolotherapy is considered as safe and effective as in adults in children [27, 45]. Although the majority of children with HHT and PAVM are asymptomatic, they require continued follow-up as the PAVMs enlarge with time and may cause complications [46, 47]. It is now recommended that all PAVMs detected by CT should be treated if possible. The previous guideline of treating PAVMs with a feeding artery 3 mm or larger is no longer generally accepted [3, 20, 48].
The pre-procedural multi-disciplinary comprehensive workup typically includes a diagnosis of PAVM by contrast echocardiography or CT pulmonary angiography, electrocardiography to rule out left bundle branch block (LBBB), serum creatinine to prevent contrast nephrotoxicity in patients with acute and chronic renal insufficiency and resting and exercise pulmonary arterial oxygen saturation [49].
a Right pulmonary angiogram is demonstrating multiple PAVMs in the right lower lobe in a 60-year-old female patient with multiple bilateral PAVMs. The patient subsequently underwent three sessions of transcatheter embolization with coils and microvascular plug. b Selective angiogram demonstrates the feeder vessel. c Angiogram demonstrates coil deployment in the feeding vessel. d Another PAVM demonstrated in the right basal segment of the same patient. e Post-embolization proximal angiogram demonstrates no residual PAVM
Principles of embolotherapy
The feeding artery should be occluded as close to the fistulous communication as possible to preserve normal perfusing lung parenchyma. It is essential to achieve adequate occlusion of all the feeding arteries, while the nidus or venous outflow need not be occluded. Intravenous heparin can be given during the procedure to prevent intraprocedural paradoxical embolism [44]. Holding the breath in deep inspiration aids to include the lung bases in the field of view during the arteriography and permits the evaluation of lesions at the lung bases without overlap. Also, this may also aid in straightening otherwise angled, tortuous vessels. Employing a closed flush system or the double flush technique helps to prevent air suction in the catheter and thus air embolism.
Technique of embolization
In general, treatment is performed under moderate sedation unless the patient has a condition that prevents them from tolerating the procedure, i.e., significant comorbidities, inability to protect the airway, inability to tolerate lying flat, in which case general endotracheal anesthesia can be employed.
When initially placing any vascular sheath during these procedures, it is paramount to place a filter for the flush bag attached to the sheath to prevent introducing air bubbles into the system. Following femoral vein or internal jugular vein access, a 6 Fr angled pigtail catheter/Grollman catheter (Cook, Bloomington, Indiana, USA) or a regular 6 Fr pigtail catheter is advanced into the pulmonary artery and the right or left main pulmonary artery is selected. Pulmonary arterial pressures are measured via a catheter-based transducer system (normal—25/8 mmHg, mean—15 mmHg). At our institution, selective digital subtraction angiography (DSA) in the main pulmonary arteries (half strength contrast material injected at a rate of 15 cm3/s with a total volume of 30 cm3/s while imaging at 6 frames/s) is performed in anterior–posterior (AP), ipsilateral and contralateral oblique views to identify PAVMs and determine the feeding vessels. The feeder vessels are then identified, mapped, and a coaxial system (8 Fr guiding catheter with an inner longer 6 Fr angle-tipped catheter combination such a Lumax system—Cook, Bloomington, Indiana, USA) is advanced over an exchange-length Rosen wire. After occluding a feeding artery of a PAVM, care must be taken to identify any additional feeding arteries in a complex PAVM. A more proximal selective DSA can confirm successful embolization of the PAVM (Figs. 1b and 2e). One can treat any number of PAVMs in a single session, but the complexity of the procedure, radiation dose to the patient and the amount of contrast material utilized delimit the number of PAVMs treated in a single session. The venous access sheath is removed after the procedure, and manual pressure is applied at the access site to attain hemostasis. After few hours of monitoring and recovery, the patient is discharged home. Acetaminophen is preferred over NSAIDs for immediate post-procedure pain as is associated with decreased risk of bleeding or epistaxis.
Among the various embolic materials available, coils (Fig. 2) and vascular plugs (Figs. 1 and 3) are the most commonly used for occluding PAVMs. The long-term effects of these embolic materials vary with the type and combination of materials used, the site of embolization proximal to the arteriovenous communication and the size of the feeding vessel. In general, coils are associated with a 20% recanalization rate and 5% for vascular plugs [31]. A single-center retrospective analysis study has suggested that coils in combination with a type I Amplatzer vascular plug can prevent recanalization [50]. Recently, polytetrafluoroethylene-covered nitinol plugs, or microvascular plugs (Medtronic), are being used for the treatment of PAVMs. Our experience (unpublished) suggests a recanalization rate of < 5% with these plugs. At present, no randomized control studies are comparing different embolization techniques or devices. The choice of an embolic device is based mainly on an individual or institutional preference. It should be noted that without regard to the 3 mm “rule,” studies have found up to 70% of patients may have residual untreatable PAVMs [51].
Benefits of embolization
Embolization of PAVMs has been shown to improve oxygenation, decrease shortness of breath and increase exercise tolerance [52]. It has also been reported to decrease the probability of paradoxical embolism and stroke, improves migraines, and decreases pulmonary hemorrhage. A multicenter retrospective study in 42 individuals who were less than 18 years diagnosed with PAVM reported that embolization is a safe and efficacious procedure in this age group and recommended that children with cyanosis, exercise intolerance, growth delay or previous complications from PAVM will benefit from the procedure [45].
Follow-up
Multidetector thoracic CT angiogram is recommended within 6–12 months after embolization and then approximately every 3 years after embolization [27]. In small untreated PAVMs and suspected microscopic PAVMs (e.g., detected on TTCE but not detectable on CT), the follow-up period should be determined on a case-by-case basis (approximately every 1–5 years) with CT angiogram, while considering radiation dose limitations and patient age. PAVM management requires life-long follow-up and continued vigilance observing for PAVM growth or recanalization [31, 53]. Several risk factors have been suggested for failed embolotherapy. These include the presence of gastrointestinal tract and/or hepatic arteriovenous fistulas at the time of initial diagnosis, pulmonary hypertension, large feeding artery diameter, smaller numbers of coils resulting in insufficient mechanical occlusion, lack of dense coil packing, under sizing coils or plugs, and embolization greater than 1 cm proximal to the nidus [49, 54].
Conclusion
Endovascular embolization of the feeding artery can prevent the complications associated with PAVMs. The procedure is minimally invasive, associated with minimum morbidity and high success rates. With the advancements in the development of newer techniques and embolization materials, recanalization rates of PAVMs are expected to decrease. However, given the chronic nature of the disease, affected patients need life-long follow-up for potential growth or recanalization of previously treated lesions to prevent the life-threatening complications associated with PAVMs.
References
Churton T (1896) Multiple aneurysms of pulmonary artery. Br Med J 1897(v.1):1223
Gossage JR, Kanj G (1998) Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med 158(2):643–661. https://doi.org/10.1164/ajrccm.158.2.9711041
Pollak JS, Saluja S, Thabet A, Henderson KJ, Denbow N, White RI Jr (2006) Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations. J Vasc Interv Radiol 17(1):35–44. https://doi.org/10.1097/01.Rvi.0000191410.13974.B6
Moussouttas M, Fayad P, Rosenblatt M, Hashimoto M, Pollak J, Henderson K, Ma TYZ, White RI (2000) Pulmonary arteriovenous malformations. Neurology 55(7):959
Goodenberger DM, Chakinala M (2015) Pulmonary arteriovenous malformations. In: Grippi MA, Elias JA, Fishman JA et al (eds) Fishman’s pulmonary diseases and disorders, 5th ed. McGraw-Hill Education, New York
Plauchu H, de Chadarevian JP, Bideau A, Robert JM (1989) Age-related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am J Med Genet 32(3):291–297. https://doi.org/10.1002/ajmg.1320320302
Shovlin CL, Letarte M (1999) Hereditary haemorrhagic telangiectasia and pulmonary arteriovenous malformations: issues in clinical management and review of pathogenic mechanisms. Thorax 54(8):714–729
Haitjema T, Westermann CJ, Overtoom TT, Timmer R, Disch F, Mauser H, Lammers JW (1996) Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med 156(7):714–719
Shovlin CL (2014) Pulmonary arteriovenous malformations. Am J Respir Crit Care Med 190(11):1217–1228. https://doi.org/10.1164/rccm.201407-1254CI
Shovlin CL, Sodhi V, McCarthy A, Lasjaunias P, Jackson JE, Sheppard MN (2008) Estimates of maternal risks of pregnancy for women with hereditary haemorrhagic telangiectasia (Osler-Weber-Rendu syndrome): suggested approach for obstetric services. BJOG 115(9):1108–1115. https://doi.org/10.1111/j.1471-0528.2008.01786.x
Cottin V, Plauchu H, Bayle JY, Barthelet M, Revel D, Cordier JF (2004) Pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. Am J Respir Crit Care Med 169(9):994–1000. https://doi.org/10.1164/rccm.200310-1441OC
Shovlin CL (2010) Hereditary haemorrhagic telangiectasia: pathophysiology, diagnosis and treatment. Blood Rev 24(6):203–219. https://doi.org/10.1016/j.blre.2010.07.001
Bayrak-Toydemir P, McDonald J, Markewitz B, Lewin S, Miller F, Chou LS, Gedge F, Tang W, Coon H, Mao R (2006) Genotype-phenotype correlation in hereditary hemorrhagic telangiectasia: mutations and manifestations. Am J Med Genet A 140(5):463–470. https://doi.org/10.1002/ajmg.a.31101
Nakayama M, Nawa T, Chonan T, Endo K, Morikawa S, Bando M, Wada Y, Shioya T, Sugiyama Y, Fukai S (2012) Prevalence of pulmonary arteriovenous malformations as estimated by low-dose thoracic CT screening. Intern Med 51(13):1677–1681
Cottin V, Dupuis-Girod S, Lesca G, Cordier JF (2007) Pulmonary vascular manifestations of hereditary hemorrhagic telangiectasia (rendu-osler disease). Respiration 74(4):361–378. https://doi.org/10.1159/000103205
Swinburne AJ, Fedullo AJ, Gangemi R, Mijangos JA (1986) Hereditary telangiectasia and multiple pulmonary arteriovenous fistulas. Clinical deterioration during pregnancy. Chest 89(3):459–460
McAdams HP, Erasmus J, Crockett R, Mitchell J, Godwin JD, McDermott VG (1996) The hepatopulmonary syndrome: radiologic findings in 10 patients. AJR Am J Roentgenol 166(6):1379–1385. https://doi.org/10.2214/ajr.166.6.8633451
Lundell C, Finck E (1983) Arteriovenous fistulas originating from Rasmussen aneurysms. AJR Am J Roentgenol 140(4):687–688. https://doi.org/10.2214/ajr.140.4.687
McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J et al (1994) Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 8(4):345–351. https://doi.org/10.1038/ng1294-345
White RI Jr, Lynch-Nyhan A, Terry P, Buescher PC, Farmlett EJ, Charnas L, Shuman K, Kim W, Kinnison M, Mitchell SE (1988) Pulmonary arteriovenous malformations: techniques and long-term outcome of embolotherapy. Radiology 169(3):663–669. https://doi.org/10.1148/radiology.169.3.3186989
White RI Jr, Mitchell SE, Barth KH, Kaufman SL, Kadir S, Chang R, Terry PB (1983) Angioarchitecture of pulmonary arteriovenous malformations: an important consideration before embolotherapy. AJR Am J Roentgenol 140(4):681–686. https://doi.org/10.2214/ajr.140.4.681
Pierucci P, Murphy J, Henderson KJ, Chyun DA, White RI Jr (2008) New definition and natural history of patients with diffuse pulmonary arteriovenous malformations: twenty-seven-year experience. Chest 133(3):653–661. https://doi.org/10.1378/chest.07-1949
Faughnan ME, Lui YW, Wirth JA, Pugash RA, Redelmeier DA, Hyland RH, White RI Jr (2000) Diffuse pulmonary arteriovenous malformations: characteristics and prognosis. Chest 117(1):31–38
Burke CM, Safai C, Nelson DP, Raffin TA (1986) Pulmonary arteriovenous malformations: a critical update. Am Rev Respir Dis 134(2):334–339. https://doi.org/10.1164/arrd.1986.134.2.334
Dines DE, Arms RA, Bernatz PE, Gomes MR (1974) Pulmonary arteriovenous fistulas. Mayo Clin Proc 49(7):460–465
Guttmacher AE, Marchuk DA, White RI Jr (1995) Hereditary hemorrhagic telangiectasia. N Engl J Med 333(14):918–924. https://doi.org/10.1056/nejm199510053331407
Faughnan ME, Palda VA, Garcia-Tsao G, Geisthoff UW, McDonald J, Proctor DD, Spears J, Brown DH, Buscarini E, Chesnutt MS, Cottin V, Ganguly A, Gossage JR, Guttmacher AE, Hyland RH, Kennedy SJ, Korzenik J, Mager JJ, Ozanne AP, Piccirillo JF, Picus D, Plauchu H, Porteous ME, Pyeritz RE, Ross DA, Sabba C, Swanson K, Terry P, Wallace MC, Westermann CJ, White RI, Young LH, Zarrabeitia R (2011) International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet 48(2):73–87. https://doi.org/10.1136/jmg.2009.069013
Langiulli M, Aronow WS, Das M, Salomon P, McClung JA, Kim-Schluger L, Wolf DC, Belkin RN (2006) Prevalence and prognosis of intrapulmonary shunts in patients with hepatic cirrhosis. Cardiol Rev 14(2):53–54. https://doi.org/10.1097/01.crd.0000173188.52195.7a
Aller R, Moya JL, Moreira V, Boixeda D, Cano A, Picher J, Garcia-Rull S, de Luis DA (1999) Diagnosis of hepatopulmonary syndrome with contrast transesophageal echocardiography: advantages over contrast transthoracic echocardiography. Dig Dis Sci 44(6):1243–1248
Nanthakumar K, Graham AT, Robinson TI, Grande P, Pugash RA, Clarke JA, Hutchison SJ, Mandzia JL, Hyland RH, Faughnan ME (2001) Contrast echocardiography for detection of pulmonary arteriovenous malformations. Am Heart J 141(2):243–246. https://doi.org/10.1067/mhj.2001.112682
Trerotola SO, Pyeritz RE (2010) PAVM embolization: an update. Am J Roentgenol 195(4):837–845. https://doi.org/10.2214/AJR.10.5230
Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, Plauchu H (2000) Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 91(1):66–67
Remy J, Remy-Jardin M, Wattinne L, Deffontaines C (1992) Pulmonary arteriovenous malformations: evaluation with CT of the chest before and after treatment. Radiology 182(3):809–816. https://doi.org/10.1148/radiology.182.3.1535899
Primack SL, Muller NL, Mayo JR, Remy-Jardin M, Remy J (1994) Pulmonary parenchymal abnormalities of vascular origin: high-resolution CT findings. Radiographics 14(4):739–746. https://doi.org/10.1148/radiographics.14.4.7938765
Gazzaniga P, Buscarini E, Leandro G, Reduzzi L, Grosso M, Pongiglione G, Pedrinazzi C, Lanzarini L, Portugalli V, Blotta P, Forner P, Boccardi E, Pagella F, Manfredi G, Olivieri C, Zambelli A, Danesino C, Inama G (2009) Contrast echocardiography for pulmonary arteriovenous malformations screening: does any bubble matter? Eur J Echocardiogr 10(4):513–518. https://doi.org/10.1093/ejechocard/jen317
Hofmann LV, Kuszyk BS, Mitchell SE, Horton KM, Fishman EK (2000) Angioarchitecture of pulmonary arteriovenous malformations: characterization using volume-rendered 3-D CT angiography. Cardiovasc Intervent Radiol 23(2):165–170
Remy J, Remy-Jardin M, Giraud F, Wattinne L (1994) Angioarchitecture of pulmonary arteriovenous malformations: clinical utility of three-dimensional helical CT. Radiology 191(3):657–664. https://doi.org/10.1148/radiology.191.3.8184042
Boussel L, Cernicanu A, Geerts L, Gamondes D, Khouatra C, Cottin V, Revel D, Douek P (2010) 4D time-resolved magnetic resonance angiography for noninvasive assessment of pulmonary arteriovenous malformations patency. J Magn Reson Imaging 32(5):1110–1116. https://doi.org/10.1002/jmri.22384
Ohno Y, Hatabu H, Takenaka D, Adachi S, Hirota S, Sugimura K (2002) Contrast-enhanced MR perfusion imaging and MR angiography: utility for management of pulmonary arteriovenous malformations for embolotherapy. Eur J Radiol 41(2):136–146
Schneider G, Uder M, Koehler M, Kirchin MA, Massmann A, Buecker A, Geisthoff U (2008) MR angiography for detection of pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. AJR Am J Roentgenol 190(4):892–901. https://doi.org/10.2214/ajr.07.2966
Tanabe Y, Landeras L, Ghandour A, Partovi S, Rajiah P (2018) State-of-the-art pulmonary arterial imaging—part 1. VASA 47(5):345–359. https://doi.org/10.1024/0301-1526/a000708
Taylor BG, Cockerill EM, Manfredi F, Klatte EC (1978) Therapeutic embolization of the pulmonary artery in pulmonary arteriovenous fistula. Am J Med 64(2):360–365
Terry PB, Barth KH, Kaufman SL, White RI Jr (1980) Balloon embolization for treatment of pulmonary arteriovenous fistulas. N Engl J Med 302(21):1189–1190. https://doi.org/10.1056/nejm198005223022107
Trerotola SO, Pyeritz RE, Bernhardt BA (2009) Outpatient single-session pulmonary arteriovenous malformation embolization. J Vasc Interv Radiol 20(10):1287–1291. https://doi.org/10.1016/j.jvir.2009.06.026
Faughnan ME, Thabet A, Mei-Zahav M, Colombo M, Maclusky I, Hyland RH, Pugash RA, Chait P, Henderson KJ, White RI Jr (2004) Pulmonary arteriovenous malformations in children: outcomes of transcatheter embolotherapy. J Pediatr 145(6):826–831. https://doi.org/10.1016/j.jpeds.2004.08.046
Mowers KL, Sekarski L, White AJ, Grady RM (2018) Pulmonary arteriovenous malformations in children with hereditary hemorrhagic telangiectasia: a longitudinal study. Pulmonary Circulation 8(3):2045894018786696. https://doi.org/10.1177/2045894018786696
Gefen AM, White AJ (2017) Asymptomatic pulmonary arteriovenous malformations in children with hereditary hemorrhagic telangiectasia. Pediatr Pulmonol 52(9):1194–1197. https://doi.org/10.1002/ppul.23686
Lee DW, White RI Jr, Egglin TK, Pollak JS, Fayad PB, Wirth JA, Rosenblatt MM, Dickey KW, Burdge CM (1997) Embolotherapy of large pulmonary arteriovenous malformations: long-term results. Ann Thorac Surg 64(4):930–939 (discussion 939-940)
Chamarthy MR, Park H, Sutphin P, Kumar G, Lamus D, Saboo S, Anderson M, Kalva SP (2018) Pulmonary arteriovenous malformations: endovascular therapy. Cardiovasc Diagn Therapy 8(3):338–349
Trerotola SO, Pyeritz RE (2010) Does use of coils in addition to amplatzer vascular plugs prevent recanalization? AJR Am J Roentgenol 195(3):766–771. https://doi.org/10.2214/ajr.09.3953
Shovlin CL, Jackson JE, Bamford KB, Jenkins IH, Benjamin AR, Ramadan H, Kulinskaya E (2008) Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax 63(3):259–266. https://doi.org/10.1136/thx.2007.087452
Gupta P, Mordin C, Curtis J, Hughes JM, Shovlin CL, Jackson JE (2002) Pulmonary arteriovenous malformations: effect of embolization on right-to-left shunt, hypoxemia, and exercise tolerance in 66 patients. AJR Am J Roentgenol 179(2):347–355. https://doi.org/10.2214/ajr.179.2.1790347
Swanson KL, Prakash UB, Stanson AW (1999) Pulmonary arteriovenous fistulas: Mayo Clinic experience, 1982–1997. Mayo Clin Proc 74(7):671–680. https://doi.org/10.4065/74.7.671
Remy-Jardin M, Dumont P, Brillet PY, Dupuis P, Duhamel A, Remy J (2006) Pulmonary arteriovenous malformations treated with embolotherapy: helical CT evaluation of long-term effectiveness after 2-21-year follow-up. Radiology 239(2):576–585. https://doi.org/10.1148/radiol.2391050333
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tellapuri, S., Park, H.S. & Kalva, S.P. Pulmonary arteriovenous malformations. Int J Cardiovasc Imaging 35, 1421–1428 (2019). https://doi.org/10.1007/s10554-018-1479-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-018-1479-x