Abstract
Patients with late/very-late stent thrombosis (ST) are at high risk of recurrent-ST. The mechanisms of recurrent-ST are largely unknown. The objective is to describe the 1-year optical coherence tomography (OCT) findings of patients suffering from late/very-late ST treated with intravascular imaging guided percutaneous coronary intervention (PCI). All consecutive patients with late/very-late ST undergoing intravascular imaging guided PCI were screened to undergo coronary angiography and OCT examination at 1 year. Patients were classified according to the observation of stent malapposition as most contributing cause of the ST. Thirty-four patients were included. Stent malapposition was observed in 17 (50%) and the remaining 17 cases were classified as: neoatherosclerosis (n = 9), underexpansion (n = 3) and unknown mechanism (n = 5). Patients with malapposition had a remarkable reduction of the malapposition volume (from 6.4 to 1.3 mm3; p = 0.02) during the ST procedure, but this was not fully corrected in 13 (76.5%). At 12 months, two patients of the malapposition group presented with uneventful target vessel re-occlusion. Persistent malapposition was observed in nine patients (60.0%). Major coronary evaginations (46.7 vs. 0%; p = 0.001) and uncovered struts (6.3 vs. 1.0%; p < 0.001) were also more frequent in patients with malapposition than without malapposition. None of the patients had thin-cap fibroatheroma neoatherosclerosis. Contributing causes of late/very-late ST are diverse and have different healing patterns at 12 months. Patients with stent malapposition treated with intravascular imaging guided PCI showed poor re-healing; but patients with other causes of the ST showed optimal stent healing as assessed by OCT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Definite stent thrombosis (ST) is defined when signs of thrombus formation within the stent are proven by angiography or post-mortem pathology [1]. Definite ST is a rare but devastating complication after percutaneous coronary intervention (PCI) associated with high in-hospital mortality (6%) and with high risk of recurrent ST [2]. According to the time interval since the stent implantation, ST is classified into three groups: early ST (≤ 1 month), late ST (1–12 months) and very-late ST (> 1 year) [1]. Late and very-late ST are mainly attributed to stent-related causes such as stent malapposition, lack of stent healing, neoatherosclerosis and stent underexpansion [3,4,5,6].
Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are recommended by current revascularization guidelines to explicate and correct underlying mechanical factors of the ST [7]. Intravascular imaging guidance has demonstrated its ability to reduce or correct stent malapposition and stent underexpansion avoiding the implantation of additional stents [5]. However, little is known regarding the best treatment for cases with in-stent neoatherosclerosis and for cases with other causes of ST. Moreover, the re-healing process of the stent after PCI has never been investigated.
The aim of the present study is to describe the 1-year OCT predictors of stent re-thrombosis (such as stent malapposition, lack of stent healing, in-stent thin-cap fibroatheroma neoatherosclerosis and stent underexpansion) of patients with late and very-late ST treated with intravascular imaging guided-PCI. Moreover, this study also aims to compare the 1-year OCT findings between patients with different stent-related causes of late/very-late ST observed at the ST procedure.
Materials and methods
Study design
This is a single-centre, observational, prospective study. All consecutive patients presenting with angiographic definite late and very-late ST (> 1 month after stent implantation) subsequently treated with PCI from June 2012 to June 2015 were screened to undergo coronary angiography and OCT imaging at 1 year [5]. The Supplementary Appendix summarizes the ST procedure, the inclusion and exclusion criteria and the intravascular imaging acquisitions. This study was approved by the local ethic committee of our institution and was carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
Quantitative IVUS and OCT analyses
IVUS images were analyzed with the QIvus® dedicated software (Medis, Leiden, the Netherlands); OCT images were analyzed in the proprietary offline software (LightLab Imaging®, Westford, MA, Abbott) by a dedicated core-laboratory (BARCICORE-LAB, Barcelona, Spain). Two experienced analysts performed the adjudication of the main underlying cause of late/very-late ST by reviewing the angiographic and intravascular images. The following causes were investigated: stent malapposition, in-stent neoatherosclerosis, stent underexpansion and unknown causes.
Quantitative OCT analysis was performed at 1-mm intervals. The OCT software drew the lumen area automatically. Stent area was drawn at the adluminal site of the metallic struts. The neointimal area was calculated as the difference between the stent and luminal areas. The neointimal thickness was automatically calculated from the center of the adluminal side of the strut to the lumen contour with the thickness ruler tool [8].
Qualitative IVUS and OCT analyses
Malapposition was defined as a clear separation of the metallic struts from the vessel wall in the absence of a side branch in case of IVUS. In case of OCT, a distance from the endoluminal surface of the stent strut to the vessel wall larger than the strut thickness (+ 20 µm of the OCT resolution) was also defined as malapposition. In-stent neoatherosclerosis was defined as the observation of clear intra-stent plaque core with lipid or calcium echogenic characteristics as assessed by IVUS. In case of OCT, fibroatheroma neoatherosclerotic plaques were defined as signal-poor regions with high signal-attenuation and diffuse borders. Fibrocalcified neoatherosclerotic plaques were defined as signal-poor regions with low signal attenuation and clear plaque borders [9]. Stent underexpansion was defined when the minimal stent area was ≤ 90% of the distal reference lumen area [10]. Follow-up OCT recordings were also evaluated qualitatively in order to assess the following findings: the neointima pattern at the cross-section with largest neointima area, the presence of signal-rich bands and the observation of coronary evaginations [8].
Statistical analysis
All patients included in the present study were divided into two categories according to the observation of stent malapposition as the main contributing cause of the ST: malapposition group versus other findings group. Categorical variables were presented as counts and percentages, and continuous variables as median [inter-quartile range (IQR)]. Comparisons of categorical variables were estimated with the Chi square test and comparisons of continuous variables were estimated using the Kruskal–Wallis non-parametric test. A two-sided p value ≤ 0.05 was considered statistically significant. Statistical analysis was performed with the SPSS software, version 20.0 (SPSS Inc., IL, USA).
Results
Study population and classification
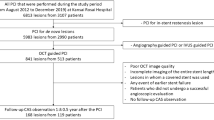
During the study period, a total of 89 patients with late and very-late ST undergoing PCI were registered out of 4.858 PCI procedures (1.8%). There were 27 early-ST (30%) and 62 late/very-late ST (70%). A total of 57 patients with late/very-late ST underwent intravascular-imaging-guided PCI and 34 of these patients underwent invasive angiographic follow-up at 12 months. At 1-year, two patients presented with complete target vessel occlusion and did not undergo OCT imaging. Figure 1 shows the flow chart of the study.
The 34 cases included were classified according to the main contributing cause of the ST as assessed by intravascular imaging prior PCI: malapposition group (n = 17) and other findings group (n = 17). The intravascular imaging causes of ST in the other findings group were: neoatherosclerosis (n = 9), stent underexpansion (= 3) and unknown causes (n = 5).
Clinical and procedural characteristics at the stent implantation procedure
Table 1 summarizes the clinical and procedural characteristics at the stent implantation procedure. Patients with stent malapposition as main contributing cause of late/very-late ST were treated with larger percentage of DES (52.9 vs. 35.3%; p = 0.380) and with smaller stents (IQR of 2.5–3.0 mm vs. 3.0–3.5 mm, respectively; p = 0.033) than patients without malapposition. Patients with malapposition had larger number of patients with hypertension (88.2 vs. 52.9%; p = 0.024).
Clinical and procedural characteristics at the ST procedure
Table 2 summarizes the clinical and procedural characteristics at the ST procedure. The median stent age was 5.8 years without differences among the study groups. Most of the patients presented with STEMI (82.4%) at the time of the ST. Additional stent implantation was used in 32.4% of patients without differences among the study groups. There were no differences regarding the largest nominal balloon/stent used to treat the late-ST (IQR of 3.00–3.75 mm) and the predicted balloon/stent diameter to the previous stent diameter ratio (IQR of 1.10–1.35). However, all patients (100%) of the malapposition group were at least with 1 antiplatelet therapy versus 76.5% of patients of the other findings group (p = 0.074).
Intravascular imaging findings at the ST procedure
A total of 34 patients with late/very-late ST were imaged with intravascular imaging techniques during the ST procedure (28 with IVUS and 6 with OCT). In total 89 pullback acquisitions were obtained (2.6 per patient). Table 1 of the Supplementary Appendix shows the quantitative findings. The reference lumen area was numerically smaller in patients with malapposition as main contributing cause of the thrombosis (IQR of 4.7–8.8 mm2) than in patients without malapposition (IQR of 6.3–10.3 mm2); p = 0.494. Mean stent area was smaller in the malapposition group (IQR of 5.1–8.1 mm2) than in the other findings group (IQR of 7.5–9.6 mm2); p = 0.033. All lumen and stent size measurements increased after balloon dilatation or additional stent implantation. Although malapposition was remarkably reduced in the malapposition group (from 6.4 to 1.3 mm3 of malapposition volume; p = 0.02), it persisted in the majority of cases (76.5%) at the end of the PCI.
Quantitative coronary angiography analysis and OCT findings at 12 months
A total of 34 patients underwent coronary angiography at 12 months. Two patients of the malapposition group presented with target vessel re-occlusion due to probable uneventful recurrent ST; and did not undergo OCT imaging. Figure 1 of the Supplementary Appendix shows the angiographic and intravascular images of those patients. Figure 2 shows the evolution of the patients according to the cause of late/very-late ST. Table 2 of the Supplementary Appendix shows the quantitative coronary angiography analysis results.
Serial intravascular imaging findings according to the main contributing cause of the late/very-late ST. ASI additional stent implantation, BA balloon angioplasty, IVUS intravascular ultrasound, MLA minimal lumen area, OCT optical coherence tomography, PCI percutaneous coronary intervention, ST stent thrombosis
Table 3 shows the OCT findings of the 32 patients imaged with OCT at 12 months. A total of 1149 cross-sections were analyzed: 155 reference distal cross-sections (13.5%), 859 in-stent cross-sections (74.8%) and 135 reference proximal cross-sections (11.7%). There were 15 in-stent cross-sections not analyzed due to blood artifacts. A total of 7790 struts were analyzed. Patients with malapposition presented with larger percentage of uncovered struts (IQR of 3.0–10.7%) than patients without malapposition (IQR of 0.5–2.3%); p < 0.001. Percentage of malapposed struts was also larger in the malapposition group (IQR of 0–4.3%) than in the other findings group (IQR of 0–0%). Patients with malapposition presented with larger number of major coronary evaginations (46.7%) than patients without malapposition (0%); p = 0.001. Intraluminal thrombus was observed in two patients of the malapposition group at 12 months (2 vs. 0; p = 0.145). Intraluminal thrombus occupied < 10% of the lumen area, had a maximal extension of 1.5–2 mm and was attached to persistent malapposed struts in both patients. Figure 2 of the Supplementary Appendix shows both cases with intraluminal thrombus. There were 16 patients with in-stent neoatherosclerosis (50.0%) without differences between the study groups. None of the neoatherosclerotic plaques were thin-cap fibroatheromas. Figures 3 and 4 shows 2 cases with malapposition and neoatherosclerosis.
Case with stent malapposition as main contributing cause of late-ST. a Very-late ST of a 2.5 × 32 mm paclitaxel-eluting stent implanted in mid-LCX 5 years ago; b IVUS image with malapposition as main cause of late ST (white arrow); c IVUS image with calcified plaque (white arrows) outside the stent and a side-branch (transparent arrow). d Angiographic result after 3.0 × 15 mm non-compliant balloon angioplasty; e, f IVUS images after balloon angioplasty, without stent malapposition; g 12 month follow-up angiography; h, i uncovered struts (#) as assessed by OCT at 12 month follow-up. *Intracoronary wire with acoustic and light shadow
Case with in-stent neoatherosclerosis as cause of late-ST. a Very-late 3.5 × 22 mm BMS thrombosis (8 years after implantation) in mid-RCA; b hyper-echogenic core (transparent arrow) with acoustic shadow hampering the visualization of the stent struts (probable neoatherosclerotic calcified plaque); c hypo-echogenic core (white arrow) corresponding to neoatherosclerotic lipid core plaque as main contributing cause of the very-late ST; d final result after PCI with a 4.0 mm non-compliant balloon angioplasty of the ST; e, f IVUS images after balloon angioplasty showing large lumen area. g Elective 1 year angiography; h, i OCT images showing large lumen area with neoatherosclerotic calcified plaque (transparent arrow) and thick-cap fibroatheroma plaque at 1 year (white arrow). *Intracoronary wire with acoustic and light shadow
Discussion
The main findings of the present study are: (1) Intravascular imaging techniques are able to discern most of the stent-related contributing causes of late/very-late ST; (2) Stent malapposition is the most common finding in patients with late/very-late ST, and is numerically more frequent with DES; (3) Neoatherosclerosis is the second cause of late-ST, and is numerically more frequent with bare-metal stents (BMS); (4) The stent healing pattern differs according to the pre-existing main contributing cause of the ST, as assessed by OCT, at 1 year; (5) Patients with late/very-late ST attributed to stent malapposition present with high-risk features for recurrent ST, such as, large amount of uncovered struts, coronary evaginations and persistent malapposed struts at 1 year, despite the use of intravascular imaging guidance at the ST; (6) In contrast, patients with neoatherosclerosis, underexpansion or no evident cause of late/very-late ST present with low-risk healing pattern of recurrent ST as assessed by OCT at 12 months.
The cumulative incidence of late/very-late ST is around 0.8% with BMS, 0.7–1.5% with first-generation DES and 0.5–0.7% with second-generation DES at 2–3 years follow-up [11]. Although its incidence decreases 5 years after the stent implantation, there are documented cases up to 20 years after the stent implantation [12, 13]. Therefore, it is plausible that PCI for late-ST will be performed more frequently in the following years.
So far, there is no consensus of the recommended treatments in case of angiographic definite late/very-late ST. Current revascularization guidelines recommend the use of intravascular imaging techniques to assess the mechanisms of stent failure (recommendation class IIa; level C) [7]. Thrombus aspiration and use of glycoprotein IIb/IIIa inhibitors have been associated with better procedural outcomes and lower risk of recurrent thrombosis [14]. In contrast, additional stent implantation has been related with worse cardiac outcomes as compared to no additional stent implantation [2, 15, 16]. Most of the clinical outcomes observed in patients treated with additional stent were related to cardiac death or recurrent ST at mid-term follow-up [2].
The present study is the first investigation of the healing process after intravascular imaging-guided PCI for late/very-late ST. According to the present study, malapposition is the most common contributing cause of late/very-late ST. By reviewing the intravascular images of the ST procedure, there were four cases (23.5%) with suspected positive vessel remodeling causing late-acquired stent malapposition. It is noteworthy that patients with malapposition had numerically smaller stent diameters than patients with other underlying substrates of late/very-late ST; despite all patients had similar reference lumen areas during the ST procedure and at 1-year follow-up. Therefore, it is plausible that the main contributing cause of the malapposition was stent under-size during the stent implantation. Moreover, although larger number of intravascular imaging acquisitions, larger number of balloon dilatations and larger predicted balloon to stent diameter ratio were performed, it is noteworthy that malapposition was not fully corrected in 76.5% of cases (of the malapposition group) during the ST procedure. Nevertheless, the amount of malapposition volume was reduced > 75% from pre-intervention to post-intervention during the ST procedure in this group, as assessed by intravascular imaging. It is probable that caution to prevent coronary perforation and thrombus embolization by aggressive balloon dilatation might have withheld the operators to fully correct the malapposition in the majority of cases. In addition, it was unknown whether leaving mild stent malapposition could be re-healed after PCI of the ST procedure. According to previous serial OCT studies including elective patients, the healing process after DES implantation is capable to integrate most of the “acute” malapposed struts into the vessel wall at 1-year follow-up [17, 18]. Maximal incomplete strut apposition distances < 270 µm after drug-eluting stent implantation were grossly covered and reapposed to the vessel wall at 6 months; only huge malapposition distances (≥ 850 µm) were associated with persisting malapposed and uncovered struts at 6 months [17]. According to the present study including patients with ST, residual malapposition persisted in 60% of patients and re-healing of persistent malapposition was not observed in most of the cases at 1-year follow-up. In fact, two patients of the malapposition group presented with asymptomatic target vessel re-occlusion at 12 months; two patients presented with intraluminal thrombus in OCT cross-sections with malapposed struts at 12 months; and three patients with persistent stent malapposition underwent new PCI with balloon angioplasty at 1-year. Therefore, more aggressive dilatations and most extensive correction of the malapposition should have been advisable during the ST procedure.
It is well known that OCT is the best intravascular imaging technique to visualize the stent healing, apposition and neoatherosclerosis [5, 7]. However, the OCT signal is unable to visualize structures behind red thrombus and can ignore the main causes of the ST in cases with persistent thrombus after intensive thrombus aspiration or balloon dilatation [3]. IVUS has poorer resolution compared to OCT. However, its use in ST is not limited by thrombus. Moreover, IVUS enables visualization of the elastic external membrane (EEM) and therefore identification of positive vessel remodeling after stent implantation by comparing the largest in-stent EEM with the EEM of the reference non-stent segments [19]. Therefore, IVUS and OCT imaging in patients with ST are useful and give complementary information. In the present study, it is possible that the five patients with no evident stent-related cause of late/very-late ST, imaged with IVUS, had underlying mild malapposition or unseen neoatherosclerosis since it was evident at follow-up OCT.
The present study has several limitations. First, this is an observational study and the number of patients included is low. Therefore, all comparisons performed between the study groups must be interpreted with caution and are hypothesis generating. Second, there was no specific protocol for the treatment of ST and all decisions were left to the operator´s discretion. Finally, the assessment of the main contributing causes of ST with two different intravascular imaging techniques may have important variability, specially in cases with in-stent neoatherosclerosis.
Conclusions
Stent-related main contributing causes of late/very-late ST are diverse and have different healing patterns, as assessed by OCT, at 12 months. In cases with stent malapposition as main underlying substrate of ST, the observation of uncovered struts, major coronary evaginations and persistent malapposed struts is frequent at 12 months. In those cases, intravascular-imaging guided procedures reduced the amount of malapposition at the ST procedure, but the re-healing of the stent was not able to correct the remaining lack of strut coverage/malapposition. In contrast, cases with in-stent neoatherosclerosis, stent underexpansion or no evident main contributing cause of late/very-late ST, the 1-year OCT healing pattern indicates a low risk of recurrent ST. Although further investigations are required, patients with malapposition as main contributing cause of late/very-late ST warrants watchful follow-up of these cases.
References
Cutlip DE, Windecker S, Mehran R et al (2007) Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 115:2344–2351
van Werkum JW, Heestermans AA, de Korte FI et al (2009) Long-term clinical outcome after a first angiographically confirmed coronary stent thrombosis: an analysis of 431 cases. Circulation 119:828–834
Souteyrand G, Amabile N, Mangin L et al (2016) Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur Heart J 37:1208–1216
Taniwaki M, Radu MD, Zaugg S et al (2016) Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation 133:650–660
Gomez-Lara J, Salvatella N, Gonzalo N et al (2016) IVUS-guided treatment strategies for definite late and very late stent thrombosis. EuroIntervention 12:e1355–e1365
Adriaenssens T, Joner M, Godschalk T et al (2017) Optical coherence tomography findings in patients with coronary stent thrombosis: a report of the PREvention of Late Stent Thrombosis by an Interdisciplinary Global European Effort (PRESTIGE) Consortium. Circulation 136:1007–1021
Authors/Task Force m, Windecker S, Kolh P et al (2014) ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 35:2541–2619
Gomez-Lara J, Brugaletta S, Jacobi F et al (2016) Five-year optical coherence tomography in patients with st-segment-elevation myocardial infarction treated with bare-metal versus everolimus-eluting stents. Circ Cardiovasc Interv 9:e003670
Taniwaki M, Windecker S, Zaugg S et al (2015) The association between in-stent neoatherosclerosis and native coronary artery disease progression: a long-term angiographic and optical coherence tomography cohort study. Eur Heart J 36:2167–2176
Russo RJ, Silva PD, Teirstein PS et al (2009) A randomized controlled trial of angiography versus intravascular ultrasound-directed bare-metal coronary stent placement (the AVID Trial). Circ Cardiovasc Interv 2:113–123
Tada T, Byrne RA, Simunovic I et al (2013) Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv 6:1267–1274
Acibuca A, Gerede DM, Vurgun VK (2015) Bare-metal stent thrombosis two decades after stenting. Cardiovasc J Afr 26:e19–e21
Yamaji K, Raber L, Zanchin T et al (2016) Ten-year clinical outcomes of first-generation drug-eluting stents: the Sirolimus-Eluting vs. Paclitaxel-Eluting Stents for Coronary Revascularization (SIRTAX) VERY LATE trial. Eur Heart J 37:3386–3395
Waldo SW, Armstrong EJ, Yeo KK et al (2013) Procedural success and long-term outcomes of aspiration thrombectomy for the treatment of stent thrombosis. Cathet Cardiovasc Interv 82:1048–1053
de la Torre-Hernandez JM, Alfonso F, Hernandez F et al (2008) Drug-eluting stent thrombosis: results from the multicenter Spanish registry ESTROFA (Estudio ESpanol sobre TROmbosis de stents FArmacoactivos). J Am Coll Cardiol 51:986–990
de la Torre Hernandez JM, Alfonso F, Gimeno F et al (2010) Thrombosis of second-generation drug-eluting stents in real practice results from the multicenter Spanish registry ESTROFA-2 (Estudio Espanol Sobre Trombosis de Stents Farmacoactivos de Segunda Generacion-2). JACC Cardiovasc Interv 3:911–919
Gutierrez-Chico JL, Wykrzykowska J, Nuesch E et al (2012) Vascular tissue reaction to acute malapposition in human coronary arteries: sequential assessment with optical coherence tomography. Circ Cardiovasc Interv 5(S1–S8):20–29
Shimamura K, Kubo T, Akasaka T et al (2015) Outcomes of everolimus-eluting stent incomplete stent apposition: a serial optical coherence tomography analysis. Eur Heart J Cardiovasc Imaging 16:23–28
Guagliumi G, Sirbu V, Musumeci G et al (2012) Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc Interv 5:12–20
Funding
This study was funded by Abbott Vascular Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors declare conflict of interest with the present study.
Ethical approval
All procedures were in accordance with the ethical standards of the local ethic´s committee and with the 1964 declaration of Helsinki and its later amendments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ñato, M., Gomez-Lara, J., Romaguera, R. et al. One-year optical coherence tomography findings in patients with late and very-late stent thrombosis treated with intravascular imaging guided percutaneous coronary intervention. Int J Cardiovasc Imaging 34, 1511–1520 (2018). https://doi.org/10.1007/s10554-018-1372-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-018-1372-7