Abstract
Using data from a commercial pressure wire system (St. Jude Medical) we previously developed an automated “smart” algorithm to determine a reproducible value for minimum FFR (smFFR) and confirmed that it correlated very closely with measurements made off-line by experienced coronary physiology core laboratories. In this study we used the same “smart” minimum algorithm to analyze data derived from a different, commercial pressure wire system (Philips Volcano) and compared the values obtained to both operator-defined steady state FFR and the online automated minimum FFR reported by the pressure wire analyser. For this analysis, we used the data collected during the VERIFY 2 study (Hennigan et al. in Circ Cardiovasc Interv, doi:10.1161/CIRCINTERVENTIONS.116.004016) in which we measured FFR in 257 intermediate coronary stenoses (mean DS 48%) in 197 patients. Maximal hyperaemia was induced using intravenous adenosine (140 mcg/kg/min). We recorded both the online minimum FFR generated by the analyser and the operator-reported steady state FFR. Subsequently, the raw pressure tracings were coded, anonymised and 256/257 were subjected to further off-line analysis using the smart minimum FFR (smFFR) algorithm. The operator-defined steady state FFR correlated well with smFFR: r = 0.988 (p < 0.001), average bias 0.008 (SD 0.014), 95% limits of agreement −0.020 to 0.036. The online automated minimum FFR also correlated well with the smFFR: r = 0.998 (p < 0.001), average bias 0.004 (SD 0.006), 95% limits of agreement −0.016 to 0.008. Finally, the online automated minimum FFR correlated well the operator-reported steady state FFR: r = 0.988 (p < 0.001), average bias 0.012 (SD 0.014), 95% limits of agreement −0.039 to 0.015. In 95% of lesions studied (244/256), the operator reported steady-state FFR, smFFR, and online automated minimum FFR agreed with each other to within 0.04, which is within the previously reported test/retest limits of agreement of FFR reported by an experienced core lab. Disagreements >0.05 among methods were rare but in these cases the two automated algorithms almost always agreed with each other rather than with the operator-reported value. Within the VERIFY 2 dataset, experienced operators reported a similar FFR value to both an online automated minimum (Philips Volcano) and off-line “smart” minimum computer algorithm. Thus, treatment decisions and clinical studies using either method will produce nearly identical results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fractional flow reserve (FFR) is a well validated index of ischemia which is measured during maximal hyperemia usually achieved during peripheral or intracoronary adenosine administration. FFR adoption has increased by approximately 300% in recent years, and now stands at 30% relative to PCI volume in the United States [1] with improving uptake across Europe according to a recent survey [2]. Despite this, global uptake has continued room for growth given the clearly demonstrated benefits to patient outcomes [3–5]. In an effort to facilitate interpretation of physiology data, efforts have focused on improving the user interface in order to optimize workflow [6]. A particular challenge exists in trying to discard spurious data which can be caused by artifacts from a variety of sources (such as aortic or pressure wire noise, signal distortion, or arrhythmia) [7]. Steady State FFR refers to FFR measured when the Pd/Pa curve displays relatively stable values over a period of analysis during hyperemia which may not always be as low as the minimal FFR value observed during the complete physiology study. These values can be selected by the operator prospectively as per the VERIFY 2 Trial [8] or an automated algorithm can be applied which looks for sequential high quality measurements and then calculates the so called “smart” minimum FFR (smFFR) which was recently described Johnson et al. [9]. The smFFR algorithm has been validated against Core Laboratory FFR and found to produce equivalent results using an automated software [9].
In this study we aimed to compare both operator defined steady state and minimal FFR versus core lab derived smFFR as well as automatically detected FFR as displayed by the Philips Volcano s5® console (Volcano FFR). We quantified reclassification of vessels among operator-defined steady state and minimum FFR, console-reported FFR, and smFFR .
Methods
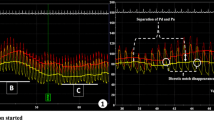
All vessels which comprised the VERIFY 2 dataset were eligible for analysis. As all patients had already consented to anonymized analysis of their data no further consent was obtained beyond that which was acquired for the main study. The methodology of this study has been described elsewhere [8] but in brief, patients with angiographically intermediate coronary stenoses (30–80% diameter stenosis) in which FFR measurement was clinically indicated were eligible to be included. All physiology data was acquired by or under the supervision of BH or SW to minimize inter-observer variation. This was a single center study performed in Golden Jubilee National Hospital, Glasgow, U.K. Standard exclusion criteria for pressure wire studies applied and included the following: severe calcific coronary disease, severe tortuosity rendering pressure wire studies difficult or impossible, recent myocardial infarction within the previous 72 h, ongoing unstable chest pain, known intolerance of adenosine or severe asthma. A Prestige® or Verrata® Wire (Philips Volcano) was inserted into the guide catheter, calibrated and passed to the distal third of the coronary artery beyond the lesion of interest. Once in position and following the administration of intra-coronary isosorbide dinitrate (200 mcg) patients had adenosine administered via large bore cannulae at a rate of 140 mcg/kg/min for a minimum of 2 min. The operating cardiologist interpreted results during the index procedure and both the minimal FFR value (excluding artifact) as well as the steady state FFR value were noted on a standardized case report form. Results as reported by the Philips Volcano software were manually extracted from the study files. Following completion of enrollment, the study data was anonymized and electronically transferred for analysis and calculation of smFFR using an automated algorithm at the Weatherhead PET Center For Preventing and Reversing Atherosclerosis, Houston, Texas. Details and results from the smFFR algorithm have been previously published [9] but, in brief, pressure signals from the aorta and coronary artery were analysed to identify beats of sufficient quality. Segments of the tracing that did not meet quality criteria (for example, loss of pressure wire signal or aortic pressure damping) were excluded. The Pd/Pa trend line was computed and averaged for valid portions of the tracings, and its lowest value defines the smart minimum FFR. Figure 1 visually summarizes the transformation of raw data into a Pd/Pa trendline.
SmFFR example raw tracing with analysis (reproduced with permission [7]). Left phasic aortic pressure (Pa) (red) and distal coronary pressure (Pd) (blue) tracings. An intravenous infusion of adenosine at 140 μg/kg/min began after approximately ten cardiac cycles and continued for the duration of the recording. Right analysis of the phasic data shows the average Pa (red) and Pd (blue) pressures for each cardiac cycle, as well as derived heart rate (green) and Pd/Pa (black). Vertical dashed gray lines denote the hyperemic period. Horizontal bars mark the smart minimum fractional flow reserve (FFR) and its associated pressures and heart rate. Note the stability of Pd/Pa during the hyperemic period [coefficient of dispersion (COD) 1.5%] compared with the much wider variations in aortic pressure (COD 7.6%), coronary pressure (COD 7.5%), and heart rate (COD 7.2%)
Statistical analysis was performed (BH) using version 23 SPSS, IBM Corp, Armonk, NY and reviewed by an independent biostatistician (JM) not involved in other parts of the study. Values were correlated using Spearman’s coefficients and the mean values were compared using paired t tests. The level of agreement between tests was examined using Bland Altman plots using Graphpad Prism ver. 6.
Results
Data from 256 of the 257 vessels was analyzable using the smFFR algorithm. Summary demographics are shown in Table 1. Means and standard deviations of FFR measured by all methods were similar and are shown in Table 2.
Spearman’s correlation coefficients are shown in Table 3. Although the FFR values derived by each modality are numerically similar there is a statistically significant difference between all pairs (Table 4) except minimal and Volcano derived FFR (p = 0.752).
The operator-defined steady state FFR correlated and agreed well with the smart minimum FFR (Fig. 2): r = 0.984 (p < 0.001), average bias 0.008 (SD 0.014), 95% limits of agreement −0.02 to 0.036 (Fig. 3, panel a). The Volcano FFR also correlated well and agreed well with the smart minimum FFR: r = 0.997 (p < 0.001), average bias 0.004 (SD 0.006), 95% limits of agreement −0.016 to 0.008 (Fig. 3, panel b). The Volcano FFR correlated well and agreed well with the operator-reported steady state FFR: r = 0.985 (p < 0.001), average bias 0.012 (SD 0.014), 95% limits of agreement 0.039 to −0.015 (Fig. 3, panel c). The minimal FFR value reported by the operator correlated well and agreed well with the Volcano FFR with an r value of 0.997, average bias of 0.0001 (SD 0.007), 95% limits of agreement −0.0134–0.0137 (Fig. 3, panel d).
a Bland Altman of smart minimum FFR versus steady state FFR. 95% limits of agreement indicated by solid lines. Average bias indicated by dashed line. b Bland Altman of smart minimum FFR versus volcano derived FFR. 95% limits of agreement indicated by solid lines. Average bias indicated by dashed line. c Bland Altman of steady state FFR versus volcano derived FFR. 95% limits of agreement indicated by solid lines. Average bias indicated by dashed line. d Bland Altman of volcano derived FFR versus minimal FFR value. 95% limits of agreement indicated by solid lines. Average bias indicated by dashed line. Note that there is the same number of points on all graphs but some have graphs have more points with identical averages and differences and these get overlaid on one another
The number of lesions considered to be functionally significant (FFR ≤ 0.80) by each parameter were as follows: 108/256 (42.2%) using the operator-defined steady state FFR, 123 (48%) using the Volcano FFR, 121 (47%) using the minimal FFR values and 115 (45%) using the smFFR algorithm. When a cutoff of ≤0.75 is applied for ischemic FFR the numbers of vessels categorized as ischemic are 57 (22.3%) for Steady State FFR, 71 (27.7%) for Volcano FFR, 71 (27.7%) for minimum FFR and 65 (25.4%) for smFFR.
In 244/256 lesions [95%, CI (92, 98)%], the operator reported that steady-state FFR, smFFR, and Volcano FFR agreed with each other to within 0.04, which is within the previously reported test/retest limits of agreement of FFR reported by an experienced core lab [9].
Discussion
We have shown that high quality physiology data obtained by experienced interventional cardiologists is similar to that obtained by using the “smart” minimum algorithm in a core laboratory setting with a reclassification in only 7/256 [2.7%, CI (1.1, 5.6)%] vessels under study between the operator defined steady state FFR and smFFR (Fig. 4). No cases were excluded in the primary analysis of VERIFY 2 [8] in order for the dataset to represent a reaI world population and similarly we only excluded 1/257 of the VERIFY 2 cases in this analysis due to technical difficulty in applying the algorithm. It is also clearly evident that the Philips Volcano algorithm reports very similar data to the operator determined minimal FFR value. Our prior study has demonstrated that 40% of patients may not reach a “steady state” during IV adenosine infusion [9]. These scenarios almost certainly occur due to insufficient adenosine delivery as a result of the long and dynamic pathway between peripheral IV infusion and central coronary artery action, especially considering effects of variable venous return, altered pulmonary transit due to tachypnoea, and red blood cell deactivation of adenosine. As a result, the lowest FFR value derived from valid data reflects the peak action of adenosine vasodilation and hence maximal hyperemia. However, the lowest FFR value without quality checking, as currently reported by several commercial platforms including the Boston Scientific Comet Wire™/Polaris™ system (Boston Medical, Marlborough, USA) and Opsens Medical system (Québec, Canada), does not reflect valid physiology. It is therefore vital that improvements with pressure wire technology are paralleled by improvements in software design which incorporates data filtering through modalities such as the smFFR algorithm. With improved confidence in reliability, less experienced operators may be more comfortable in making decisions based on physiological data especially when FFR values are close to ischemic cut-offs.
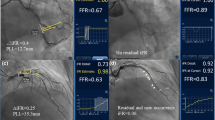
Pd/Pa during rest and hyperemia during a coronary physiology study. smFFR is indicated by the black line (0.782) and operator defined steady state FFR is indicated by the dotted red line (0.82). In this case there was a significant discordance between measurements which would have led to a reclassification as ischemic if the smFFR measurement was adopted
Limitations of this study
This is a single center study from a unit that performs high volume coronary physiology as part of local and international studies. This may mean that operator experience may not be generalizable to other centers and there is therefore potential that the difference in smFFR and operator-defined FFR values may be underestimated in this study. Furthermore, our prior work measured FFR twice [10] which provided an independent method to judge values based on test/retest similarity unlike the single measurements obtained in the current study.
Conclusions
Smart minimum FFR results are similar to operator-defined FFR values when they are specifically sought and recorded in experienced coronary physiology centers. Using the automated minimal values on pressure wire consoles results in reclassification of up to 6% of cases as ischemic when a cut-off value of ≤0.80 is utilized and up to 5% change in reclassification as ischemic when a ≤0.75 cut-off is adopted. Operators must ensure careful observation for artifact during recordings.
Future directives
Future use of algorithms to improve catheterization laboratory workflow will be important in determining the usefulness of the “smart” minimum algorithm in everyday practice. Furthermore, software/hardware that provides automated recognition of drift during a case as well as exclusion of measurements with pressure damping or other artifacts would all serve to improve the quality of physiology data. Clearly there are a large number of patients whose FFR data falls within the FFR grey-zone whose results are open to interpretation depending on the institutional/operator ischemic cut-off and whether minimal or steady state values are applied. Future prospective studies may provide interesting data on the prevalence of ischemia in this group to aid treatment strategies (NCT02425969).
References
Desai NR, Bradley SM, Parzynski CS et al (2015) Appropriate use criteria for coronary revascularization and trends in utilization, patient selection, and appropriateness of percutaneous coronary intervention. JAMA 314:2045–2053
Tebaldi M, Biscaglia S, Pecoraro A, Fineschi M, Campo G (2016) Fractional flow reserve implementation in daily clinical practice: a European survey. Int J Cardiol 207:206–207
Pijls NHJ, Fearon WF, Tonino PAL et al (2010) Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease. J Am Coll Cardiol 56:177–184
Pijls NHJ, van Schaardenburgh P, Manoharan G et al (2007) Percutaneous coronary intervention of functionally nonsignificant stenosis. J Am Coll Cardiol 49:2105–2111
De Bruyne B, Fearon WF, Pijls NHJ et al (2014) Fractional flow reserve–guided PCI for stable coronary artery disease. N Engl J Med 371:1208–1217
Nijjer SS, Sen S, Petraco R et al (2014 Dec) Pre-angioplasty instantaneous wave-free ratio pullback provides virtual intervention and predicts hemodynamic outcome for serial lesions and diffuse coronary artery disease. J Am Coll Cardiol Cardiovasc Interv 7(12):1386–1396
Tarkin JM, Nijjer S, Sen S et al (2013) Hemodynamic response to intravenous adenosine and its effect on fractional flow reserve assessment: results of the Adenosine for the Functional Evaluation of Coronary Stenosis Severity (AFFECTS) Study. Circ Cardiovasc Interv 6:654–661
Hennigan B, Oldroyd KG, Berry C (2016)et al. Discordance between resting and hyperemic indices of coronary stenosis severity: the VERIFY 2 Study (A comparative study of resting coronary pressure gradient, instantaneous wave-free ratio and fractional flow reserve in an unselected population referred for invasive angiography). Circ Cardiovasc Interv. doi:10.1161/CIRCINTERVENTIONS.116.004016
Johnson NP, Johnson DT, Kirkeeide RL et al (2015) Repeatability of fractional flow reserve despite variations in systemic and coronary hemodynamics. J Am Coll Cardiol Cardiovasc Interv 8:1018–1027
Berry C, van’t Veer M, Witt N, Kala P, Bocek O, Pyxaras SA et al (2013) VERIFY (VERification of Instantaneous Wave-Free Ratio and Fractional Flow Reserve for the Assessment of Coronary Artery Stenosis Severity in EverydaY Practice): a multicenter study in consecutive patients. J Am Coll Cardiol 61(13):1421–1427
Acknowledgements
We thank our patients, medical, nursing and cardiac physiology staff who supported this study. Dr. Hennigan was supported by British Heart Foundation Project Grant PG/14/97/31263 and an institutional grant from the British Heart Foundation RE/13/5/30177 to the University of Glasgow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclosures
There was no industry involvement in any aspect of this study. Professor Berry has undertaken research, consulting and lectures for St. Jude Medical based on contracts with The University of Glasgow. Professor Oldroyd has received honoraria for consultancy and lectures from St. Jude Medical and Volcano Corporation. NPJ receives internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis; and significant institutional research support from St. Jude Medical (for NCT02184117) and Volcano/Philips Corporation (for NCT02328820), makers of intracoronary pressure and flow sensors. His institution (UTHealth) has a licensing agreement with Boston Scientific for the smFFR algorithm.
Rights and permissions
About this article
Cite this article
Hennigan, B., Johnson, N., McClure, J. et al. Validation of the “smart” minimum FFR Algorithm in an unselected all comer population of patients with intermediate coronary stenoses. Int J Cardiovasc Imaging 33, 991–997 (2017). https://doi.org/10.1007/s10554-017-1126-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-017-1126-y