Abstract
Overproduction of thyroid hormones leads to structural as well as functional cardiac changes. Conventional echocardiography but also recently developed sophisticated two-dimensional echocardiography speckle (2D-STE) tracking allow elaborate evaluation of these changes. Our purpose was to investigate the effects of thyroid hormones overproduction on the heart in patients with Graves’ disease and changes that occur after 6 months thyrostatic therapy. We conducted a prospective, case-control study of 6 months duration. Full echocardiographic assessment at diagnosis and after 6 months of thyrostatic therapy were performed in 44 patients with Graves’ disease, aged 37.6 ± 9.1 years. Additionally, 43 euthyroid controls were studied for the same time period. Left ventricular end diastolic diameter (LVEDD) and left ventricular end systolic diameter (LVESD) were higher in the patient group while triscupid annular plane systolic excursion (TAPSE) was lower in the patient group. Moreover, left ventricular (LV) mass index and left atrium (LA) volume index were higher in the Graves’ disease group. Diastolic impairment as assessed with conventional echocardiography including tissue Doppler was present in the patient group (E/A ratio 0.87 ± 0.10,). 2D-STE analysis, revealed an increase in the strain rate at the isovolumic relaxation time (SRIVRT, 0.310 ± 0.07 patients versus 0.298 ± 0.09 s−1 controls). Improvement in diastolic and right systolic function as well as in left ventricular structural parameters was observed after restoration of euthyroidism (E/A ratio from 0.87 ± 0.10 versus 0.9 ± 0.08, p < 0.05). Patients with newly diagnosed Graves’ showed an improvement in diastolic function, right systolic function and structural parameters after 6 months of thyrostatic treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Graves’ disease is an autoimmune disorder that usually develops in young and middle-aged patients leading to severe hyperthyroidism. Hyperthyroidism influences the cardiovascular system and results to increased heart rate due to increased sympathetic tone as well as increased stroke volume due to increased preload and increased afterload [1, 2]. Moreover, thyroid hormones affect left ventricular (LV) structure leading, in longstanding hyperthyroidism, to concentric cardiac hypertrophy [3, 4].
These changes were until recently measured with conventional echocardiographic methods, but newer techniques, such as Tissue Doppler and Two Dimensional-Speckle Tracking Echocardiography (2D-STE) can provide a more detailed imaging OF the structural and functional changes that occur during the thyroxin excess [5].
Although there are several studies addressing the effect of thyroid hormones on the heart in subclinical hyperthyroidism (SH) [2, 3, 6–10] there are only a few in patients with overt hyperthyroidism and even fewer solely in patients with Graves’ disease [2, 4, 11–14].
Therefore the aim of the present study was to investigate the effects of thyroid overproduction on the heart in Graves’ disease patients and changes that may occur after 6 months thyrostatic therapy by assessing both conventional echocardiographic parameters and multidirectional strain assessed by 2D-STE.
Subjects and methods
Study population
The present study was a prospective case-control study of 6 months duration with two parallel groups. 43 healthy subjects (Controls: 20 male, 23 female; 38 ± 10.6 years, mean ± sd), 44 patients with overt hyperthyroidism due to Graves’ disease (Patients: 20 male, 24 female; 37.6 ± 9.1 years, mean ± sd), were included in the study. Patients were newly diagnosed with overt Graves’ disease confirmed by low TSH levels and elevated, FT4 and elevated TSH receptor antibody. Thyroid malignancy was excluded in all patients by the use of echocardiography and nodule testing by fine needle aspiration.
All patients received thyrostatic therapy with carbimazole (n = 30) or methimazole (n = 14) 10–20 mg according to the severity of the hyperthyroidism.
Control subjects were healthy volunteers with normal thyroid function tests.
Physical examination, hormonal assessments and echocardiographic evaluation were performed at baseline at 3 months and at 6 months follow up. Changes in LV hemodynamics and performance were evaluated by conventional echocardiography and by sophisticated 2D-STE analyses. All echocardiographic analyses were performed by a single observer, blinded to study group.
Inclusion criteria included the presence of newly diagnosed overt hyperthyroidism due to Graves’ disease, age >18 years and having signed the patient consent form.
Exclusion criteria included a history of coronary artery disease (CAD), diabetes mellitus, hypertension, left ventricular wall motion abnormality, history of structural heart disease, valvular heart disease, anaemia, electrolyte disorders, renal failure, pulmonary disease, connective tissue disease and one (n = 1) patient was excluded for poor imaging quality. None of the participants used any thyrostatic medication before their inclusion in the study. Additionally no drugs known to influence cardiovascular parameters were allowed.
The study protocol was conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the institutional review board of Aristotle University of Thessaloniki. All participants gave their written informed consent.
Patients were recruited from the outpatient clinic of the Department of Endocrinology of AHEPA and 1st Cardiology Department, AHEPA University Hospital, Thessaloniki, Greece.
Protocol
Assessment of thyroid status
The evaluation of thyroid morphology and function was performed by clinical examination. Measurement of serum free thyroid hormones (FT3 and FT4) and thyroid stimulating hormone (TSH) levels was conducted using the commercial Immulite kits (IMMULITE 2000 XPI, Siemens Healthcare, Erlangen, Germany). The normal reference intervals for FT3 was 1.8–5.2 ng/dL, for FT4 was 0.80–1.9 ng/dL, and for TSH was 0.40–4.0 mIU/mL. Hyperthyroidism was diagnosed clinically and confirmed by high FT3 and/or FT4 concentrations with suppressed serum TSH concentrations.
Echocardiographic examination
Patients were imaged in the left lateral decubitus position.
All subjects underwent two-dimensional, M-mode, pulsed, and colour flow Doppler echocardiographic examinations with a commercially available machine (Vivid 7 Pro, GE, 2–4 MHz phased-array transducer) by a cardiologist who was blind to the clinical details and results of the other investigations of each patient and control group. During echocardiography, 1 lead electrocardiogram was recorded continuously.
Data were acquired triggered to the QRS complex and saved in cine loop format for off-line analysis (EchoPac 108.1.5, General Electric/Vingmed Ultrasound).
M-mode measurements and conventional Doppler echocardiographic examinations were performed according to the criteria of the American Society of Echocardiography (ASE) guidelines [15]. LV diastolic function was evaluated by mitral inflow velocities, namely peak E (early diastolic) and peak A (late diastolic), E/A ratio and also by deceleration time of the E-wave (DT) and isovolumic relaxation time (IVRT). All measurements were recorded as an average of 3 cardiac cycles. Left atrium (LA) dimension, LV end-systolic and end-diastolic dimensions, diastolic ventricular septal thickness, and diastolic thickness of LV posterior wall were measured in parasternal long-axis view. LV ejection fraction (EF) was estimated by Simpson’s rule. LV mass was calculated with the Devereux Formula and indexed to body surface. LA volume was measured by the method of discs in the apical four-chamber view and indexed to the body surface area (BSA) [16].
According to the recommended ASE guidelines for the right heart assessment the following dimensions were measured: RV basal apical four-chamber view, RV wall thickness (subcostal long axis view), proximal RVOT (parasternal short axis view at the great vessels level), distal RVOT (parasternal long axis view), Furthermore, RV function was evaluated by measuring the tricuspid annular plane systolic excursion (TAPSE), Dp/Dt, and fractional shortening [17]. TAPSE which is a simple method of estimating RV function is measured as the distance among the tricuspid annulus and RV apex at the end-diastole and end-systole of the same cardiac cycle. Its lower reference value for impaired RV systolic function is 16 mm.
Two-dimensional Fractional Area Change is one of the recommended methods of quantitatively estimating RV function, with a lower reference value for normal RV systolic function of 35 %.
RV dP/dt < approximately 400 mm Hg/s is likely abnormal.
Tissue Doppler imaging (TDI)
Tissue Doppler imaging uses Doppler principles to measure the velocity of myocardial motion [18]. TDI was performed with transducer frequencies of 3.5–4.0 MHz, adjusting the spectral pulsed Doppler signal filters until a Nyquist limit of 15–20 cm/sec, and using the minimal optimal gain.
Since TDI is angle-dependent cautious was taken to place the myocardial area of interest in the center of the ultrasound beam for parallel alignment of motion plane with the cursor.
Myocardial velocities named as Sm (peak systolic), Em (early diastolic), and Am (late diastolic) were obtained at the lateral mitral and lateral tricuspid annulus. LV Em/Am, LV E/Em, and RV Em/Am ratios were calculated. The cases with LV Em/Am < 1 were accepted to have LV diastolic dysfunction.
Two-dimensional speckle tracking strain echocardiography
Speckle tracking echocardiography is based on an analysis of the spatial dislocation (tracking) of speckles which are small particles, (natural acoustic markers), on two-dimensional images. Speckles are equally distributed within the myocardium and visible in the standard grayscale.
Strain, expressed as percentage (%), is a measure of myocardial deformation in 3 spatial directions: longitudinal, radial and circumferential, derived by tracking the displacement of speckles frame-to-frame during the cardiac cycle. Strain rate indicates the rate of change in strain, and is usually expressed as sec-1.
Longitudinal, radial and circumferential strain were analysed by a single investigator blinded to therapy (K.A.) using the Automated Function Imaging (AFI) software on a commercially available machine (GE Vivid 7 Dimension, Norway).The frame rate was kept between 70 and 100 Hz. All the subjects underwent conventional echocardiography and then STE (2D strain) using speckle tracking software (Echo Pac, GE Healthcare). Strain values of 6 segments of the LV were measured.
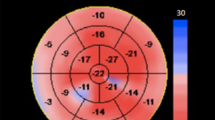
Longitudinal strain represents myocardial deformation directed from the base to the apex. During systole myocardial fibers shorten with a translational and represent a base-to-apex directed myocardial deformation. Longitudinal strain was measured at the apical four-chamber, two-chamber and long axis views and global values derived from the average of the 18 LV segmental peak strain values. (Fig. 1a). Radial strain stands for myocardial deformation directed radially toward the center of the LV cavity and circumferential strain reflects the LV myocardial shortening along the circular perimeter observed in short-axis view. Both, radial and circumferential strain were assessed at the mid ventricular LV short-axis view and global values derived from the average of the 6 segmental LV peak strain values (Fig. 1b, c). Finally, strain rate at the isovolumic relaxation time (SRIVRT), a marker of global myocardial relaxation, was measured at the three LV apical views and averaged for final analysis (Fig. 1d).
Two-dimensional speckle tracking strain imaging, strain and strain rate values Strain values of 6 segments of the LV were measured at the apical four-chamber, two chamber and long axis views for longitudinal strain (a) and at the mid ventricular short axis view for radial (b) and circumferential strain (c). SR IVRT, a marker of global myocardial relaxation, is measured at the three LV apical views (d). The white arrow indicate the value of strain rate at the isovolumic relaxation time.
Statistical analysis
All statistical analyses were performed with SPSS software (version 16.0, SPSS Inc., Chicago, IL, USA).
Normality of distributions was ascertained using Kolmogorov–Smirnov test. Parametric variables are expressed as mean ± sd. Non parametric variables were expressed as median (range).
Pearson’s r or Spearman test was used for parametric and non parametric correlations respectively. Student’s t test was used for mean comparison of parametric variables and Mann Whitney test if otherwise distributed.
Chi square test was used to determine whether there is significant difference between the expected frequencies and the observed frequencies for categorical variables.
Repeated measures analysis of variance (ANOVA) was used to investigate differences in the biochemical and echocardiographic variables from baseline to the 3-month and 6-month time points.
For non parametric variables we used the Friedman test respectively.
Univariate regression analysis was used to define significant relations between a.FT4 and b.TSH with echocardiographic parameters at baseline.
A p value of <0.05 was considered statistically significant.
Results
Table 1 presents the baseline demographics of the Graves’ disease group (n = 44) and of the control group (n = 43). The mean age of the population was 37.6 ± 9.1 for the patients versus 38.6 ± 10.6 years for the controls (p = 0.26). At baseline systolic blood pressure (BP) and diastolic BP were comparable (141 ± 15.1 versus 136 ± 16.6 mmHg, p = 0.39) and (79.1 ± 9.1 versus 80.1 ± 9.9 mmHg, p = 0.65, mean ± sd) for patients and controls respectively. BMI was also comparable in the two groups. Electrolytes and basic biochemistry measurements were normal in both groups.
Heart rate was significantly higher in the Graves’ disease group compared with the controls (101.7 ± 21.7 versus 79.6 ± 9.1 bpm, p = 0.018). All patients were in normal sinus rhythm.
Similarly free T3 and free T4 were also significantly higher in the Graves’ disease group compared to controls (11.2 ± 5.8 versus 5.22 ± 2.8 pg/mL, p < 0.001 and 33.9 ± 11.0 versus 17.3 ± 2.8 ng/dL, p < 0.001). TSH, as expected was significantly lower in the patients (0.065 (0.99) versus 1.87 (5.39) mIU/L, p < 0.001, median (range)).
As a result of the thyrostatic treatment 29 (66 %) Graves’ disease patients eventually achieved euthyroidism in a 6 months follow up.
After treatment there was a significant reduction in FT3, FT4, and a significant increase in TSH levels in the patient group. Values of these parameters in the control group did not differ between baseline and 6 months (Table 2).
Graves’ patients versus controls-baseline conventional echocardiography and 2D-STE analysis
At baseline all measured echocardiographic parameters were within the reference ranges in all studied subjects (Table 3). Furthermore, LV dimensions, wall thickness and LV mass index were increased in the patient group compared with controls. Regarding the RV function TAPSE and dp/dt were significantly reduced compared to the controls. LVEF was significantly lower in the patient compared to the control group but the difference lost significance after adjusting for heart rate.
As for the diastolic function the patient group showed significantly lower values of E and A wave with an inverse E/A ratio and significantly delayed E-wave DT and IVRT, which indicate impaired LV relaxation.
Similarly, the tissue Doppler analysis showed an inverse E′/A′ ratio. Finally 2D-STE namely LV radial, circumferential and longitudinal strains and SRIVRT were comparable between the two groups and were within normal values according to the meta-analysis of normal ranges of left ventricular strain [19] (Table 5).
Conventional echocardiography and 2D-STE analysis after 6 months of thyrostatic therapy
At 6 months of receiving the thyrostatic treatment, the Graves’ disease patients demonstrated a significant decrease in LVEDD, LV mass index, LA volume index and LA diameter whereas LV ejection fraction (LVEF) did not change significantly during the course of therapy. On the other hand TAPSE and dp/dt showed a small improvement.
There was an improvement of LV diastolic function including a reduction in A wave, A′ wave and E/A ratio but the inversion of E/A ratio remained.
In tissue Doppler analysis there were similar results (Table 4).
As for the 2D-STE analysis, circumferential strain was lower in the patient group at the end of the study. However, SRIVRT significantly increased in the patient group (Table 5).
Relationship of FT4 and TSH levels with echocardiographic parameters at baseline
Univariate regression analysis revealed a strong correlation of FT4 levels and IVST and a significant correlation with TAPSE (Table 6). Moreover TSH levels related to LV systolic dimension but not to diastolic. There was no correlation with FT4 and TSH levels and LV diastolic parameters as measured with tissue Doppler imaging. Finally, SRIVRT, as measured with 2D-STE analysis, showed a borderline association with FT4 levels.
Discussion
To our knowledge this is the first study to analyze the influence of thyroid hormone excess on left and right ventricular function and structure in patients with newly diagnosed Graves’ disease using the novel 2D-STE.
The majority of studies with echocardiographic evaluation in thyroid patients are performed in patients with subclinical hyperthyroidism, where the cardiovascular effects included diastolic dysfunction and increased LVM [2, 3, 6–10]. In an extensive literature search we found only few studies with conventional echocardiographic evaluation of cardiac systolic and diastolic function in patients with Graves’ hyperthyroidism [2, 4, 11–14].
One of the findings in many studies with overt hyperthyroidism is the diastolic dysfunction [4, 20–22]. In accordance our study revealed impaired diastolic function as assessed with conventional pulsed wave Doppler echocardiography and tissue Doppler imaging. After restoration of euthyroid hormone levels the diastolic function slightly improved. 2D-STE revealed an important finding, namely the increase in SRIVRT after thyrostatic therapy. Improvement of the LV filling pattern with an increase in SRIVRT provides additional information as this parameter is less load-dependent and relies on active deformation of the myocardium. Moreover, it was significantly and inversely correlated with FT4 only in the patient group which strengthens the aforementioned finding.
Although most studies with overt hyperthyroidism report increased contractility and LVEF [11–14] in studies with Graves’ disease patients this phenomenon seems to be different. Tsymbaliuk et al., showed a lower LVEF in patients with Graves’ disease compared to controls which was improved by treatment [23]. Oliveros Ruiz et al., propose an impaired reversibility of LVEF in patients with thyrotoxic cardiomyopathy [24]. Similarly we found a lower LVEF in Graves’ disease patients and no improvement after treatment. However, differences between the two groups lost significance after adjusting for heart rate. A proposed explanation for this is that patients with severe hyperthyroidism may develop “high-output heart failure”, which may be due to tachycardia-mediated cardiomyopathy [11]. Cardiac output is increased although congestive circulation is present. Subsequently, congestive heart failure, systolic or diastolic, may be the initial presentation in patients with primary hyperthyroidism [25].
Moreover, circumferential strain in the patient group was significantly higher compared to the control group but unchanged from baseline while radial and longitudinal strain showed no differences which delineate that there were no significant changes in systolic function after treatment.
Further, we reported increased LVM, although within reference ranges, in patients with Graves’ disease which is in line with most studies with patients with overt hyperthyroidism [26–28]. Increased thyroid hormone action simulates the cardiac protein synthesis in cardiac myocytes [3] and leads to cardiac hypertrophy causing increase in LV mass (LVM). Moreover, we presented a strong positive correlation between FT4 level and the IVST. Harun S. on the other hand, who studied the correlation between thyroxine level and LVM in 17 Graves’ disease patients found no correlation [18]. It seems that the duration of exposure to high thyroxine level and the homogeneity as well as the sample of the patient population studied may play a role.
Regarding the right heart structure, we found a significant increase in RV and RVOT dimensions and regarding RV function a significant reduction in TAPSE and fractional area reflecting reduced RV contractility [17]. After 6 months of thyrostatic treatment improvement of RV function was documented.
Our study had some limitations that should be mentioned. CAD was not excluded based on coronary angiography but solely on patient history. However, we included young patients without cardiovascular risk factors, thus with low expected prevalence of CAD. Moreover we did not assess pulmonary artery pressures that may also be an early sign of hyperthyroidism [17]. Furthermore, in the present study we did not include a patient group with subclinical hyperthyroidism since we wanted to focus on the cardiac Graves’ patients solely to have a plain message. However this could be the subject of another study that could describe the changes in cardiac function across the spectrum of hyperthyroidism.
One of our strong points is that the study population was purely Graves’ disease patients while patients with toxic adenoma and toxic multinodular goiter were excluded to keep our study population homogeneous. The autoimmune origin of Graves’ disease discriminates the disease from other hyperthyroidism states. Autoantibodies may affect the myocardium on top of the thyroxin excess. In this context it should be studied as an entity in regards to its effects in organ systems and not within the general spectrum of hyperthyroidism.
In conclusion, contrary to other forms of hyperthyroidism, Graves’ disease may manifest with an impaired systolic function as was shown by our study as well as other studies. We also found a mild diastolic dysfunction that marginally improved after therapy without however a clear clinical significance. 2D speckle tracking validated the findings of the conventional echocardiography that with the major strain indexes (radial and longitudinal) remaining unchanged after treatment.
Other studies are needed to further investigate Graves’ disease as a separate entity, from subclinical and other forms of clinical hyperthyroidism, in regards to the structure and function of the myocardium.
References
Klein I, Danzi S (2007) Thyroid disease and the heart. Circulation 116:1725–1735
Pearce EN, Yang Q, Benjamin EJ, Aragam J, Vasan RS (2010) Thyroid function and left ventricular structure and function in the Framingham Heart Study. Thyroid 20:369–373
Dillmann W (2010) Cardiac hypertrophy and thyroid hormone signaling. Heart Fail Rev 15:125–132
Klein I, Ojamaa K (2001) Thyroid hormone: targeting the vascular smooth muscle cell. Circ Res 88:260–261
Mondillo S, Galderisi M, Mele D et al (2011) Speckle-tracking echocardiography: a new technique for assessing myocardial function. J Ultrasound Med 30:71–83
Dorr M, Ittermann T, Aumann N et al (2010) Subclinical hyperthyroidism is not associated with progression of cardiac mass and development of left ventricular hypertrophy in middle-aged and older subjects: results from a 5-year follow-up. Clin Endocrinol (Oxf) 73:821–826
Iqbal A, Schirmer H, Lunde P et al (2007) Thyroid stimulating hormone and left ventricular function. J Clin Endocrinol Metab 92:3504–3510
Kaminski G, Michalkiewicz D, Makowski K et al (2011) Prospective echocardiographic evaluation of patients with endogenous subclinical hyperthyroidism and after restoring euthyroidism. Clin Endocrinol (Oxf) 74:501–507
Smit JW, Eustatia-Rutten CF, Corssmit EP et al (2005) Reversible diastolic dysfunction after long-term exogenous subclinical hyperthyroidism: a randomized, placebo-controlled study. J Clin Endocrinol Metab 90:6041–6047
Abdulrahman RM, Delgado V, Hoftijzer HC et al (2011) Both exogenous subclinical hyperthyroidism and short-term overt hypothyroidism affect myocardial strain in patients with differentiated thyroid carcinoma. Thyroid 21:471–476
Biondi B (2012) Mechanisms in endocrinology: heart failure and thyroid dysfunction. Eur J Endocrinol 167:609–618
Hadzovic-Dzuvo A, Kucukalic-Selimovic E, Nakas-Icindic E et al (2010) Echocardiographic evaluation of cardiac function in female patients with thyroid disorders. Bosn J Basic Med Sci 10:112–115
Mintz G, Pizzarello R, Klein I (1991) Enhanced left ventricular diastolic function in hyperthyroidism: noninvasive assessment and response to treatment. J Clin Endocrinol Metab 73:146–150
Roef GL, Rietzschel ER, Van Daele CM et al (2014) Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid 24:223–231
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–270
Devereux RB, Reichek N (1977) Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 55:613–618
Rudski LG, Lai WW, Afilalo J et al (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23:685–713
Harun S (2006) The correlation between free thyroxine levels and left ventricular mass in Graves’ disease. Acta Med Indones 38:193–195
Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH (2013) Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr 26:185–191
Sokmen A, Acar G, Sokmen G et al (2013) Evaluation of atrial electromechanical delay and diastolic functions in patients with hyperthyroidism. Echocardiography 30:1194–1201
Yue WS, Chong BH, Zhang XH et al (2011) Hyperthyroidism-induced left ventricular diastolic dysfunction: implication in hyperthyroidism-related heart failure. Clin Endocrinol (Oxf) 74:636–643
Petretta M, Bonaduce D, Spinelli L et al (2001) Cardiovascular haemodynamics and cardiac autonomic control in patients with subclinical and overt hyperthyroidism. Eur J Endocrinol 145:691–696
Tsymbaliuk I, Unukovych D, Shvets N, Dinets A (2015) Cardiovascular complications secondary to graves’ disease: a prospective study from ukraine. PLoS One 10:e0122388
Oliveros-Ruiz L, Vallejo M, Diez Canseco LF, Cardenas M, Hermosillo JA.(2013) Determinants of thyrotoxic cardiomyopathy recovery. Biomed Res Int 2013:452709
Berlin T, Lubina A, Levy Y, Shoenfeld Y (2006) Graves’ disease presenting as right heart failure. Isr Med Assoc J 8:217–218
Marcisz C, Jonderko G, Wroblewski T, Kurzawska G, Mazur F (2006) Left ventricular mass in patients with hyperthyroidism. Med Sci Monit 12:CR481–CR486
Merillon JP, Passa P, Chastre J, Wolf A, Gourgon R (1981) Left ventricular function and hyperthyroidism. Br Heart J 46:137–143
Nixon JV, Anderson RJ, Cohen ML (1979) Alterations in left ventricular mass and performance in patients treated effectively for thyrotoxicosis. A comparative echocardiographic study. Am J Med 67:268–276
Funding
This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Author contributions
Aroditis K: Acquisition of data and drafting the article. Pikilidou M: Data analysis, interpretation of the data and drafting the article. Vourvouri EC: Interpretation of the data and drafting the article. Hadjistavri L: Critical revision of the article. Zebekakis P: Critical revision of the article, final approval of the version submitted. Yovos J: Critical revision of the article, final approval of the version submitted. Efthimiadis G: Conception and design of the study, final approval for submission. Karvounis C: Conception and design of the study, final approval for submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no Conflicts of interest.
Rights and permissions
About this article
Cite this article
Aroditis, K., Pikilidou, M., Vourvouri, E. et al. Changes in cardiac function and structure in newly diagnosed Graves’ disease. A conventional and 2D-speckle tracking echocardiography study. Int J Cardiovasc Imaging 33, 187–195 (2017). https://doi.org/10.1007/s10554-016-0984-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-0984-z