Abstract
Pulmonary vascular resistance (PVR) is an important hemodynamic parameter in patients with heart failure, especially when the pulmonary arterial pressure is lower due to reduced stroke volume. Several echocardiographic methods to estimate PVR have been proposed, but their applications in patients with organic left-sided heart diseases have been limited. The aim of the present study was to examine the usefulness of our new method to estimate PVR (PVRPR) based on the continuous-wave Doppler velocity measurements of pulmonary regurgitation in these patients. In 43 patients who underwent right heart catheterization, PVRPR was calculated as the difference between the Doppler-derived early- and end-diastolic pulmonary artery (PA)-right ventricular (RV) pressure gradients divided by the cardiac output measured in the left ventricular outflow tract by echocardiography. The PVRPR correlated well with invasive PVR (PVRCATH) (r = 0.81, p < 0.001) without any fixed bias in Bland–Altman analysis. The conventional echocardiographic PVRs showed inadequate correlations with PVRCATH, or a obvious overestimation of PVRCATH. In the receiver operating characteristic analyses to determine the patients with abnormal elevation of PVRCATH (>3 Wood units, WU), the area under the curve was the greatest for PVRPR (0.964) compared to the conventional PVRs (0.649–0.839). PVRPR had 83 % sensitivity and 100 % specificity at the optimal cut-off value of 3.10 WU in identifying patients with PVRCATH >3 WU. Our simple and theoretical PVRPR is useful for the noninvasive estimation of PVR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The evaluation of pulmonary artery (PA) pressure plays an important role in the diagnosis and follow-up of patients with various cardiopulmonary diseases [1]. However, PA pressure is blood flow-dependent, and decreases due to a reduction in cardiac output. In this setting, pulmonary vascular resistance (PVR) more accurately reflects the patient’s condition than PA pressure. PVR is measured by right heart catheterization as the difference between the mean PA pressure and the PA wedge pressure divided by the cardiac output. However, the invasive nature of catheterization makes it difficult or impossible to perform daily or repetitive measurements of PVR. Although several noninvasive methods to estimate PVR based on echocardiographic measurements have been proposed [2–7], these methods used various empirical constants and/or ignored the left ventricular filling pressure, and thus their accuracy may be limited. The guidelines of the American Society of Echocardiography recommended not to use noninvasive estimations of PVR as a substitute for the invasive evaluation of PVR [8]. It has been reported that the early-diastolic and end-diastolic pressure gradients between the PA and the right ventricle (RV) derived from pulmonary regurgitation (PR) velocities measured by using continuous-wave Doppler echocardiography can reflect the mean PA pressure and the PA wedge pressure, respectively [7–10]. We therefore postulated that, by using these parameters, we could establish a simpler and more accurate noninvasive method to estimate PVR. This method uses the formula which is almost the same as the original one and includes the parameter reflecting left atrial pressure, and does not use any empirical constant. The aim of the present study was to examine the usefulness of our new method to estimate PVR based on the measurements of the early- and end-diastolic PA-RV pressure gradient by continuous-wave Doppler echocardiography in patients with left heart disease.
Methods
Patients
The study subjects were 43 patients who underwent right heart catheterization and echocardiography under stable clinical condition within 1 day (range 2–30 h, mean and SD 21 ± 7 h, median 22 h) between January 2013 and December 2014 in the Department of Cardiovascular Medicine, Hokkaido University. Patients with atrial fibrillation and those with inadequate right heart catheterization pressure tracing were excluded. Patients with severe tricuspid regurgitation were also excluded because of potential inaccuracy of cardiac output measured by the thermodilution method. The study subjects consisted of 29 males and 14 females, and their ages ranged from 20 to 88 years (59 ± 17 years old). They had ischemic cardiac disease in 20, cardiomyopathy in 11, valvular heart disease in 7 (3 with aortic stenosis, 2 after aortic valve replacement, 1 with mitral regurgitation and 1 after mitral valve repair), and other cardiovascular diseases in 5. Cardiac catheterization was performed for assessment of coronary artery stenosis in 23, hemodynamic assessment of heart failure in 14, diagnosis of structural heart disease in 5 and postoperative evaluation in 1. Among our 43 patients, 13 had pulmonary hypertension (mean PA pressure ≥25 mmHg) and were exclusively classified into the Group 2 (pulmonary hypertension due to left heart disease) according to the European Society of Cardiology and the European Respiratory Society (ESC/ERS) guidelines [1]. This study was approved by the Research Ethics Committee of Hokkaido University Hospital as a retrospective observational study.
Right heart catheterization
Right heart catheterization was performed using a water-filled catheter for hemodynamic measurements. The PA systolic pressure, PA diastolic pressure, mean PA pressure and PA wedge pressure were measured. Cardiac output was determined by the thermodilution method, and the PVR (Wood units, WU) was calculated using the following equation:
The abnormal elevation of PVRCATH was defined as >3 WU according to the ESC/ERS guidelines [1].
Echocardiographic examination
Echocardiography was performed using an Aplio XG/Artida equipped with a 2.5/3.0 MHz probe (Toshiba Medical Systems, Otawara, Japan), an E9 with an M5S probe (GE Healthcare, Little Chalfont, Buckinghamshire, UK), or an iE 33with an S5 probe (Phillips, Einthoven, Netherlands). Basic echocardiographic parameters were measured according to the guidelines of the American Society of Echocardiography (ASE) [11]. Regional wall motion was scored using a standard 16-segment left ventricular model. Each segment was scored using a 4-point scale as follows: 1 = normal, 2 = hypokinesis, 3 = akinesis, and 4 = dyskinesis. Tricuspid annular plane systolic excursion was measured in the apical four-chamber view. The peak early-diastolic transmitral flow velocity (E) and the early-diastolic mitral annular velocity at the interventricular septal side (septal e′) were measured, and the ratio of E to e′ (E/septal e′) was calculated.
The flow velocity in the RV outflow tract was recorded by placing the sample volume at the level of the pulmonary valve annulus in the parasternal short-axis view, and the time velocity integral (TVIRVOT), acceleration time and ejection time were measured. The peak tricuspid regurgitant velocity (TRV) was measured as the highest of the velocities obtained from the lower parasternal and apical multiple views. RV isovolumic contraction time was obtained as the interval between the onset of tricuspid regurgitation and that of RV ejection flow, and the total systolic time was obtained as the sum of the isovolumic contraction time and ejection time [2].
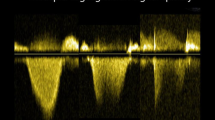
The PR flow velocity was recorded using continuous-wave Doppler echocardiography. In order to minimize the incident angle of the beam to a PR jet, the color flow signal of PR jet was visualized in two mutually orthogonal planes from several different left parasternal echo windows, and the PR flow velocity was recorded from the echo window providing the least incident angle. The peak early-diastolic and end-diastolic velocities of PR were measured to calculate the early- and end-diastolic PA-RV pressure gradients using the simplified Bernoulli equation (Fig. 1). The cardiac output was calculated from the time velocity interval of the left ventricular ejection flow and the diameter of the left ventricular outflow tract.
Measurement of pulmonary regurgitant velocities. A continuous-wave Doppler recording of pulmonary regurgitation is shown. The peak early-diastolic velocity (V1) and end-diastole velocity (V2) were measured to calculate the early- and end-diastolic pulmonary-right ventricle pressure gradient, respectively
A contrast enhancement technique was not used in the continuous-wave Doppler recording. Every measurement was done in a cardiac cycle during a breath hold at shallow expiration or at the intermediate expiratory position under quiet respiration by a single observer (S. K.) blinded to the cardiac catheterization data.
We calculated the echocardiographic PVR (WU) reported by Scapellato et al. [2], that by Abbas et al. in 2003 [3], that by Dahiya et al. [4], that by Lindqvist et al. [5], that by Abbas et al. in 2013 [6], and that by our new method (PVRPR) using the following equations:
where RV systolic pressure = 4 × TRV2 + estimated right atrial pressure
where mean PA pressure = (4 × TRV2 + 10) × 0.61 + 2
Statistical analyses
Statistical analyses were performed using standard statistical software (SPSS II for Windows, SPSS, Chicago, IL, USA). All numerical data were presented as a mean ± SD. The relationship between variables was examined using a linear correlation and a regression analysis. A Bland–Altman analysis was done to assess a systematic error of echocardiography compared to right heart catheterization [12]. Wilcoxon rank sum test was used to compare continuous variables. A receiver operating characteristic analysis was used to evaluate the accuracy of echocardiographic PVR values for the discrimination of patients with an abnormal elevation of PVRCATH. For all statistical tests, p values <0.05 were considered significant. Intraobserver and interobserver variabilities were assessed in 20 randomly selected subjects by one observer (S. K.) twice and by a second observer (M. M.).
Results
The clinical, hemodynamic and basic echocardiographic characteristics of the studied patients are presented in Table 1. Out of the 43 study patients, we could measure PVR-Scapellato in 40 patients (93 %), PVR-Abbas-2003 in 36 patients (84 %), PVR-Dahiya in 35 patients (81 %), PVR-Lindqvist in 36 patients (84 %), PVR-Abbas-2013 in 36 patients (84 %) and PVRPR in 38 patients (88 %). TRV could be measured in 36 patients (84 %), peak early-diastolic PR velocity in 38 (88 %) and end-diastolic PR velocity in 39 (91 %).
Figure 2 shows the results of the correlation and regression analyses between the echocardiographic PVRs and PVRCATH, and Fig. 3 provides the results of Bland–Altman analyses. The correlation coefficient was 0.49 (p = 0.001) in PVR-Scapellato, 0.54 (p = 0.001) in PVR-Abbas-2003, 0.54 (p = 0.001) in PVR-Dahiya, 0.76 (p < 0.001) in PVR-Lindqvist, 0.66 (p < 0.001) in PVR-Abbas-2013 and 0.81 (p < 0.001) in PVRPR. The correlation with PVRCATH was best for PVRPR, followed by PVR-Lindqvist.
Correlation and regression analyses between the pulmonary vascular resistance (PVR) obtained using catheterization (PVRCATH) and the PVRs estimated using echocardiography. a Echocardiographic PVR reported by Scapellato et al., b Abbas et al. in 2003, c Dahiya et al., d Lindqvist et al., e Abbas et al. in 2013, and f by our method (PVRPR)
Bland–Altman analyses between the pulmonary vascular resistance (PVR) obtained using catheterization (PVRCATH) and PVRs estimated using echocardiography. a Echocardiographic PVR reported by Scapellato et al., b Abbas et al. in 2003, c Dahiya et al., d Lindqvist et al., e Abbas et al. in 2013, and f by our method (PVRPR)
Although PVRPR was slightly greater than PVRCATH (2.23 ± 1.33 vs. 1.97 ± 1.15 WU, p = 0.03), no fixed bias was revealed by the Bland–Altman analysis (mean 0.25; 95 % limits of mean agreement, −0.01 to 0.51). On the other hand, PVR-Lindqvist was significantly greater than PVRCATH (3.58 ± 3.06 vs. 1.98 ± 1.17 WU, p < 0.001) and had a direct fixed bias (mean 1.61; 95 % limits of mean agreement, 0.85–2.36) showing distinct overestimation of PVR by this method.
In the receiver operating characteristic analyses to determine the patients with the abnormal elevation of PVRCATH (>3 WU), the area under the curve was the greatest for PVRPR (0.964) compared to PVR-Scapellato (0.697), PVR-Abbas-2003 (0.789), PVR-Dahiya (0.648), PVR-Lindqvist (0.839) and PVR-Abbas-2013 (0.789). PVRPR had 83 % sensitivity and 100 % specificity at the optimal cut-off value of 3.10 WU in identifying patients with PVRCATH >3 WU (Fig. 4).
Receiver operating characteristic curves to discriminate patients with pulmonary vascular resistance (PVR) obtained using catheterization (PVRCATH) >3 WU by the echocardigraphic PVRs. a Echocardiographic PVR reported by Scapellato et al., b Abbas et al. in 2003, c Dahiya et al., d Lindqvist et al., e Abbas et al. in 2013, and f by our method (PVRPR). Auc was the greatest (0.964) for PVRPR by our method compared to other previous methods (0.649–0.839). AUC area under the curve
Intraobserver and interobserver measurements for PVRPR showed interclass correlation coefficients of 0.92 and 0.90, respectively, indicating satisfactory reproducibility of the measurement of PVRPR.
Discussion
The results of the present study demonstrated that, in patients with the organic left-sided heart diseases, our new method using continuous-wave Doppler-derived PR velocities could more accurately estimate PVRCATH compared to the previous representative methods using echocardiography. Among the echocardiographic PVRs, PVRPR had the best correlation and agreement with PVRCATH. Moreover, PVRPR could identify patients with abnormally elevated PVRCATH with 83 % sensitivity and 100 % specificity at the optimal cut-off value 3.10 WU, which is nearly equal to the catheter cut-off value of 3 WU defined in the ESC/ERS guidelines. Our method is simple; it requires measurements of only the PR velocities and cardiac output, and is consistent with the original formula being used for the invasive measurement without using any empirical constant. Moreover, by including the variable reflecting left atrial pressure, this method enabled the assessment of PVR in patients with post-capillary pulmonary hypertension associated with left-sided heart diseases, in whom the accurate assessment of PVR had been difficult by previous noninvasive methods.
Several noninvasive methods to estimate PVR based on echocardiographic measurements have been proposed previously (Table 2). However, the guidelines of the ASE recommended not to use noninvasive estimations of PVR as a substitute for the invasive evaluation of PVR [8]. In the present study, PVR-Scapellato, PVR-Abbas-2003 and PVR-Abbas-2013 inadequately correlated with PVRCATH in our patients with post-capillary pulmonary hypertension, probably due to the lack of any parameter reflecting left atrial pressure in their equation. The correlation between PVR-Dahiya and PVRCATH was not satisfactory, probably because the ability of E/e′ to estimate PA wedge pressure was not very high [13, 14]. PVR-Lindqvist showed a good correlation with PVRCATH (r = 0.76), comparable to our method (r = 0.81). However, their method distinctly overestimated PVRCATH, and this method may not be applicable to patients with post-capillary pulmonary hypertension.
It has been reported that PR velocity could be recorded by continuous-wave Doppler in 74–86 % of patients in a clinical setting [15]. In our series, both the early-diastolic and end-diastolic PR velocities, and thus the PVRPR, could be obtained in 38 of 43 patients (88 %). The measurable rate of our method was not very high, but was similar to those of the other conventional methods except for the Scapellato method. It has been reported that the early-diastolic pressure gradient derived from the peak early-diastolic PR velocity is useful for estimating the mean PA pressure [7–10], whereas the end-diastolic pressure gradient calculated from the end-diastolic PR velocity reflects end-diastolic PA pressure [7, 9]. It was also reported that the end-diastolic PA pressure measured by Doppler echocardiography correlated well with invasively measured PA wedge pressure and might be used as its surrogate [10]. Thus, in our new method, PVRPR can be calculated using a formula that is very similar to that used for the PVRCATH, without any empirical constant. Our method requires the measurements of only two velocities from a single PR velocity recording and the cardiac output in the left ventricular outflow tract, and it may be one of the simplest methods to measure PVR among the noninvasive methods reported previously.
The systolic RV-right atrial pressure gradient can be noninvasively obtained using TRV [8], and it can be used to estimate the mean PA pressure as in the first and second Abbas [3, 6], Dahiya [4], and Lindqvist methods [5]. However, a wide regurgitant orifice may develop most frequently in the tricuspid valve among the four cardiac valves, probably because of the easy distensibility of right heart structures around the tricuspid annulus [16, 17]. A very wide regurgitant orifice leads to laminar regurgitant flow instead of turbulent flow [18, 19], and the peak systolic RV-right atrial pressure gradient can be greatly overestimated by the simplified Bernoulli equation due to the pressure recovery phenomenon. Such a severe functional tricuspid regurgitation may frequently develop in patients with long-standing pulmonary hypertension. Such a situation can cause a decrease in PA pressure despite the deteriorated hemodynamics due to low flow, and it typically requires the assessment of PVR instead of PA pressure. Although an overestimation of pressure gradients can also occur in patients with severe PR, such patients may be rare and limited to those after surgery for tetralogy of Fallot. Thus, our method is considered to be especially useful to evaluate patients with both left and right heart failure.
There are several limitations in this study. First, right heart catheterization and echocardiography were not performed simultaneously, and thus the hemodynamic alternations might not be completely excluded due to the study’s retrospective nature. Second, the sample size of the present study was fairly small. Third, some skill may be required for examiners to accurately measure both the early- and end-diastolic PR velocities to estimate PVR using our method. Finally, although the PA diastolic pressure is known to correspond well with the mean PA wedge pressure in patients with left heart failure, it is also reported that the PA diastolic pressure might be significantly higher than the PA wedge pressure in patients with pre-capillary hypertension such as pulmonary arterial hypertension, pulmonary thromboembolism, lung disease and tachycardia [20]. Thus, our method may not be applicable to patients with these conditions.
Conclusion
Our new method based on the continuous-wave Doppler measurements of early- and end-diastolic PA-RV pressure gradients is useful for the noninvasive estimation of PVR in patients with left-sided organic heart diseases.
References
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barberà J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol Ç, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Völler H, Luis Zamorano J (2016) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart 37:67–119
Scapellato F, Temporelli PL, Eleuteri E, Corrà U, Imparato A, Gianuzzi P (2001) Accurate noninvasive estimation of pulmonary vascular resistance by Doppler echocardiography in patients with chronic heart failure. J Am Coll Cardiol 37:1813–1819
Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ (2003) A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol 41:1021–1027
Dahiya A, Vollbon W, Jellis C, Prior D, Wahi S, Marwick T (2010) Echocardiographic assessment of raised pulmonary vascular resistance: application to diagnosis and follow-up of pulmonary hypertension. Heart 96:2005–2009
Lindqvist P, Söderberg S, Gonzalez MC, Tossavainen E, Henein MY (2011) Echocardiography based estimation of pulmonary vascular resistance in patients with pulmonary hypertension: a simultaneous Doppler echocardiography and cardiac catheterization study. Eur J Echocardiogr 12:961–966
Abbas AE, Franey LM, Marwick T, Maeder MT, Kaye DM, Vlahos AP, Serra W, Al-Azizi K, Schiller NB, Lester SJ (2013) Noninvasive assessment of pulmonary vascular resistance by Doppler echocardiography. J Am Soc Echocardiogr 26:1170–1177
Milan A, Magnino C, Veglio F (2010) Echocardiographic indexes for the non-invasive evaluation of pulmonary hemodynamics. J Am Soc Echocardiogr 23:225–239
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23:685–713
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22:107–133
Lee RT, Lord CP, Plappert T, Sutton MS (1989) Prospective Doppler echocardiographic evaluation of pulmonary artery diastolic pressure in the medical intensive care unit. Am J Cardiol 64:1366–1370
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28:1–39
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Geske JB, Sorajja P, Nishimura RA, Ommen SR (2007) Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation 116:2702–2708
Mullens W, Borowski AG, Curtin RJ, Thomas JD, Tang WH (2009) Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation 119:62–70
Borgeson DD, Seward JB, Miller FA Jr, Oh JK, Tajik AJ (1996) Frequency of Doppler measurable pulmonary artery pressures. J Am Soc Echocardiogr 9:832–837
Tei C, Pilgrim JP, Shah PM, Ormiston JA, Wong M (1982) The tricuspid valve annulus: study of size and motion in normal subjects and in patients with tricuspid regurgitation. Circulation 66:665–671
Mikami T, Kudo T, Sakurai N, Sakamoto S, Tanabe Y, Yasuda H (1984) Mechanisms for development of functional tricuspid regurgitation determined by pulsed Doppler and two-dimensional echocardiography. Am J Cardiol 53:160–163
Minagoe S, Rahimtoola SH, Chandraratna PA (1990) Significance of laminar systolic regurgitant flow in patients with tricuspid regurgitation: a combined pulsed-wave, continuous-wave Doppler and two-dimensional echocardiographic study. Am Heart J 119:627–635
Cobey FC, Fritock M, Lombard FW, Glower DD, Swaminathan M (2012) Severe tricuspid valve regurgitation: a case for laminar flow. J Cardiothorac Vasc Anesth 26:522–524
Davidson CJ, Bonow RO (2012) Cardiac catheterization. In: Bonow RO, Mann DL, Zipes DP, Libby P (eds) Braunwald’s Heart Disease, 9th edn. Elsevier Saunders, Philadelphia, pp 383–405
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Rights and permissions
About this article
Cite this article
Kaga, S., Mikami, T., Murayama, M. et al. A new method to estimate pulmonary vascular resistance using diastolic pulmonary artery-right ventricular pressure gradients derived from continuous-wave Doppler velocity measurements of pulmonary regurgitation. Int J Cardiovasc Imaging 33, 31–38 (2017). https://doi.org/10.1007/s10554-016-0965-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-0965-2