Abstract
The association between epicardial fat and coronary artery disease (CAD) might be affected by general adiposity. We aimed to determine whether the percentage of epicardial adipose tissue (%EAT), defined as the mass ratio of epicardial fat to body fat, could improve prediction of asymptomatic CAD. We consecutively enrolled 846 adults who underwent coronary computed tomography angiography as part of a health check-up and assessed their coronary stenosis severity and epicardial fat mass. Body fat mass was measured by bioelectrical impedance analysis. Subjects with CAD history, hyperthyroidism, pitting edema, or subjects taking diuretics or thiazolidinedione were excluded. Obstructive CAD was defined as at least one coronary artery with 50 % or greater obstruction, and severe CAD was defined as 70 % or greater obstruction. The %EAT had the maximum area under the curve for predicting the presence of CAD and superior discriminative performance to EAT and other EAT-indexed parameters. Multivariable logistic regression analysis revealed that %EAT >0.41 % was a predictor of obstructive CAD [odds ratio 3.59 (95 % confidence interval 2.28–5.64)], and %EAT >0.47 % was a predictor of severe CAD [4.01 (2.01–7.99)] after adjustment for calcium score and Framingham risk score. This prediction was more pronounced in subjects with higher body fat percentage (≥25 % for men and ≥35 % for women), Framingham risk score (≥10 %), or calcium score (≥100). A spillover of body fat at epicardium over a critical threshold is associated with significant coronary stenosis. This association was independent of obesity, coronary calcium burden, and Framingham risk factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epicardial adipose tissue (EAT) has received attention in recent years for its site-specific properties, which demonstrate associations with coronary artery disease (CAD). EAT is a metabolically active visceral fat depot surrounding coronary arteries and possess greater inflammatory activity than subcutaneous fat or visceral adipose tissue [1]. Increased EAT volume, as quantified by multidetector computed tomography (MDCT), is associated with cardiovascular risk factors [2], coronary artery plaque [3], and progression of coronary calcification and atheroma [4]. However, a recent multicenter study reported no association between EAT volume, coronary calcium score, and prevalent CAD [5]. Thus, there is still uncertainty as to whether EAT is a marker of general adiposity or a coronary atherogenic fat depot [6].
One approach to define the association between EAT and CAD was to concurrently assess adiposity. Prior work has documented that the impact of body mass index (BMI) on the association is different for patients with low and high BMI [7, 8]. Notwithstanding, some studies have shown that EAT is associated with cardiac events independent of BMI [9, 10]. Thus, the impact of BMI on the association between EAT and CAD is still inconclusive. The discrepancies might be attributed to the selection of an appropriate adiposity parameter as the confounding factor in CAD prediction. Indeed, BMI classification overlooks subjects with increased cardiovascular risk factors related to elevated adiposity [11]. Among adiposity parameters, body fat mass has a stronger association with total mortality than does BMI [12, 13]. These observations prompted the conjecture that, when studying CAD, it is more appropriate to adjust for the body fat mass of individual subjects than for BMI. In this scenario, it is tempting to speculate that EAT percentage (%EAT), defined as the mass ratio of EAT to body fat, could produce more consistent results. To validate the hypothesis, we compared %EAT to other adiposity parameters in their associations with calcium score, plaque score, and prevalence of CAD at a threshold of 50 or 70 % coronary stenosis. We focused on whether adding %EAT improved prediction of stenosis degree of coronary plaque beyond the coronary calcium burden and clinical risk factors.
Methods
Study population
Study participants were consecutively enrolled after undergoing coronary CT angiography as part of a general health check-up protocol at National Taiwan University Hospital from 2008 to 2011. Our Ethics Committee approved the study protocol, and all subjects provided written informed consent. The major exclusion criteria were a history of typical angina or known CAD, serum creatinine level ≥2.0 mg/dL, known hypersensitivity to iodine-based contrast agents, and hyperthyroidism. In addition, using BIA to estimate body fat assumes that the body is within the normal hydration range. Therefore, individuals who have pitting edema, are on diuretics, or are taking thiazolidinedione were excluded in order to minimize the effects of measurement error. Structured questionnaires about family history, lifestyle habits, and medical history were collected. Anthropometric parameters such as body weight, body height, and waist circumference (WC) were measured. BMI was calculated as weight (kg)/square of height (m2). Body surface area (BSA) was calculated as [height (cm) × weight (kg)/3600]½. WC was measured at the midline between the low costal margin and superior posterior iliac crest. Blood pressure was measured in a sitting position. Diabetes mellitus was defined as a history of diabetes, being on medical treatment for diabetes, or having a measured fasting plasma glucose ≥126 mg/dL. Smoking history was classified as current smoker or nonsmoker; current smokers were defined as having regularly smoked cigarettes in the year prior to the examination. Metabolic syndrome was modified according to the National Cholesterol Education Program’s Adult Treatment Panel III criteria, which classifies abdominal obesity as a waistline of 90 cm for men and 80 cm for women. Venous blood samples were collected after an at least 12-h overnight fast. Biochemical measurements, including glucose, total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, gamma-glutamyl transpeptidase (γ-GT), and creatinine concentrations, were measured using a Hitachi 7450 automated analyzer (Hitachi, Tokyo, Japan). Hemoglobin A1c was measured using a DCA 2000 analyzer (Bayer Diagnostics, Elkhart, IN, USA). Highly sensitive C-reactive protein (CRP) level was determined using a high-sensitivity immunoturbidimetric assay (Denka Seiken, Tokyo, Japan) on a Hitachi 911 analyzer (Roche Diagnostics, Indianapolis, IN, USA). The coefficients of variation of these measurements were approximately 5 %. Homeostasis model assessment ratio (HOMA-IR), which is calculated as fasting insulin (µU/ml) × fasting plasma glucose (mg/dL)/405, was used to quantify insulin resistance [14]. The Framingham score is based on data from a sample of Framingham heart studies [15]. The defining risk factors of Framingham risk score (FRS) are age, cigarette smoking, blood pressure, total cholesterol, HDL cholesterol, and diabetes.
Body fat mass
Body fat mass was measured using a DX 300 system (Jawon Medical, Kyuangsan City, Korea) at an operating frequency of 50 kHz at 250 µA [16, 17]. Participants stood on a non-conducting surface with their arms extended and legs slightly separated for 5 min. Four electrodes and cables were attached to the right hands and ankles of the participants; when measurements stabilized, the direct resistance was displayed by the bioimpedance analyzer. The five factors of weight, height, impedance, sex, and age were used to measure body fat mass. Body fat percentage was calculated from the ratio of body fat mass to weight. Cutoff value for body fat percentage used for defining obesity was ≥25 % in men and ≥35 % in women [11]. On a random sample of 100 participants, the coefficients of variation for body fat mass and percentage were 2.3 and 1.9 %, respectively.
Multidetector computed tomography
Unless contraindicated, subjects received propranolol or diltiazem for a heart rate target of ≤65 beats per minute (bpm) and nitroglycerin 0.4 mg sublingually before image acquisition. MDCT was performed using a 64-slice scanner (LightSpeed VCT, GE Healthcare, Milwaukee, WI, USA) or a 256-slice scanner (Brilliance iCT, Philips, Eindhoven, The Netherlands). Initially, an unenhanced axial CT image of the heart was obtained with a prospectively ECG-triggered axial acquisition for the purpose of coronary artery calcium scoring (Agatston method). Then, nonionic contrast media (iopromide; Ultravist 370 mgI/mL, Bayer HealthCare) was injected using a dual-barrel injector (Stellant D, Medrad, Bayer HealthCare). Contrast-enhanced CT imaging was performed to obtain images from the mid-ascending aorta to the diaphragm. Scanning parameters for the 64-slice scanner were 64 × 0.625 mm collimation, 120 kV tube-voltage, and 0.35 s tube-rotation time, and those for 256-slice scanner were 128 × 0.625 mm collimation, 120 kV tube-voltage, and 0.27 s tube-rotation time. Tube voltage was adjusted based on subject BMI. The voltage was lowered to 100 kV for patients with a BMI ≤25 kg/m2. When the heart rate was ≤65/min, prospectively ECG-triggered axial acquisition was used, and images were obtained at 70, 75, and 80 % of the cardiac cycle. When the heart rate was >65/min, retrospectively ECG-gated spiral acquisition was used, and images were reconstructed at 40, 45, 55, 70, 75, and 80 % of the cardiac cycle. Estimated radiation dose using the dose-length product and a conversion factor (0.0140 mSv/mGy cm) was 4.5 ± 1.6 mSv with the use of prospective ECG-triggering and 14.2 ± 4.6 mSv with the use of retrospective ECG-gating.
Coronary artery plaque
Analysis of CT images was performed by an experienced investigator (LWJ) who followed the recommended quantitative stenosis grading in the SCCT Guidelines [18] and was blinded to subject clinical information. A 17-segment model of coronary arteries was used for evaluation and visual semiquantification of coronary artery plaques. In each coronary artery segment, coronary atherosclerosis was defined as the presence of >1 mm2 tissue structures composed of (1) calcified plaques that had a higher density than contrast-enhanced vessel lumen, (2) noncalcified plaques that had a lower density than contrast-enhanced vessel lumen, or (3) plaques with mixed calcified and noncalcified morphologies. We adopted the two coronary artery plaque scores constructed by Min et al. [19] to assess the severity of coronary plaques: (1) segment-stenosis score and (2) segment-involvement score. Briefly, a segment involvement score was defined as the total number of coronary artery segments exhibiting plaque, irrespective of the degree of luminal stenosis within each segment (minimum = 0, maximum = 17). Each coronary segment was graded based on the extent of coronary luminal obstruction, including normal, mild (<50 %), moderate (50–69 %), or severe (>70 %), with assignment of scores of 0, 1, 2, or 3, respectively. Segmental stenosis scores of all 17 coronary segments were summed to yield a total score ranging from 0 to 51. Segments were additionally graded by plaque composition (isolated calcified, isolated noncalcified plaque, and mixed plaque). One or more stenosis with ≥50 % diameter-stenosis in at least one major coronary artery or its branches was defined as obstructive CAD, and ≥70 % stenosis was defined as severe CAD. Calcium scores of 0, 1–100, 101–400, or >400 indicated zero, mild, moderate, or severe coronary calcification, respectively. The sensitivity, specificity, and accuracy of MDCT were 85, 100, and 92 %, respectively, to detect significantly stenotic lesions (≥50 % stenosis) using invasive coronary angiography as the standard on a per-segment analysis in 24 patients with CAD.
Epicardial adipose tissue
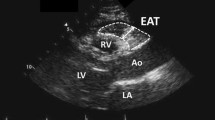
EAT was defined as the adipose tissue between the visceral pericardium and heart surface (Fig. 1). Multiplanar reconstructions of MDCT data were obtained in a standardized ventricular short-axis plane at basal, midcavity, and apical levels as well as in horizontal long-axis plane with a 3 mm slice thickness and a 2 mm slice interval. Cross-sectional area was measured on a workstation (Advantage Workstation 4.3; GE Healthcare). Adipose was identified as tissue with a density between −30 and −190 Hounsfield units. To calculate EAT volume, all axial images (approximately 200 per subject) were loaded into a workstation, and the pericardium was manually traced in these images. Based on a random sample of 60 images evaluated by two investigators, intra- and inter-observer reproducibility for EAT volume measurements were >0.95. EAT mass was defined as the product of EAT volume and fat density (0.92 g/cm3) [20]. %EAT was calculated as the ratio of EAT mass to body fat mass. BMI-indexed EAT, BSA-indexed EAT, or WC-indexed EAT was calculated as the ratio of EAT volume to BMI, BSA, or WC, respectively.
Segmentation method for the measurement of total EAT volume. a The top boundary is set at 1 cm above the superior extent of the left coronary artery (red line). b The axial cut off of the top boundary shows the presence of the right pulmonary artery. The surrounding pericardium is identified by orange arrows. c Tracing the pericardium in different multiplanar reformations. d A density range between −30 and −190 Hounsfield Units is employed to identify adipose tissue
Statistical analysis
Sample size was calculated to detect an odds ratio of 3.0 in severe CAD among the two study groups: lower %EAT and higher %EAT. Power analysis revealed a minimum of 202 participants per group with power 90 % at a significance level <0.05 for a two-sided test. Data are expressed as mean ± standard deviation or median (interquartile range). Frequencies are given as counts (%). Subjects were divided into two groups (%EAT < or ≥ the median value of %EAT) according to %EAT. Baseline characteristics were represented by lower- and higher-%EAT groups. The data were tested for normal distribution prior to utilization in the parametric analysis. The distribution of cardiovascular risk factors was assessed, and differences in group means or proportions were evaluated using Student’s t test or Pearson’s Chi-square test, as appropriate. In non-normally distributed variables, differences in group medians were assessed using a nonparametric test. Stepwise linear regression analysis was performed using EAT mass or percentage as a dependent variable and cardiovascular risk factors as independent variables. Spearman rank correlations were determined for adiposity parameters and coronary plaque scores. Area under the receiver-operating characteristic curve (AUC) analysis with non-parametric method was used to compare adiposity parameters for their ability to predict CAD. The highest value of Youden’s index was used to identify optimal cutoff points. The Stata function “roccomp” was used to test for AUC equality. Stepwise binary logistic regression analyses were performed for independent variables related to obstructive or severe CAD. The forward: likelihood ratio method was used to select variables for the final model. Multicollinearity in the logistic regression solution was detected by examining the standard errors for β coefficients. Logistic regression models were evaluated using a 75/25 % cross-validation method. The AUC analysis, integrated discrimination improvement (IDI) value, and net reclassification improvement (NRI) were used to assess the extent to which EAT indices increased the discriminative performance of CAD prediction. Forest plots were created to analyze subgroups for prediction of CAD by %EAT based on sex, calcium score, FRS, or obesity. A probability value of P < 0.05 was considered statistically significant. All calculations were performed with SPSS statistical software (SPSS 18.0 version for windows, SPSS, Chicago, IL, USA) and Stata (version 13.1; StataCorp, College Station, TX).
Results
Baseline characteristics
We consecutively enrolled 846 asymptomatic adults (70.8 % male, 56 ± 9 years old) who showed a wide range of EAT, with a mean volume of 92 cm3 (11–253 cm3), which corresponds to an EAT mass of 85 g (10–233 g) and a median %EAT of 0.41 % (0.07–1.21 %). The age of participants was older and the proportion of males was greater in the higher %EAT group (Table 1). There were significant increases in the prevalence of hypertension and diabetes mellitus and an increase in FRSs with increasing %EAT. Subjects with increased %EAT were more likely to be receiving lipid-lowering drugs. This likely accounts for the fact that correlation between higher %EAT and high total cholesterol level was not a statistically significant difference. Adiposity measurements varied with increasing %EAT. There was a significant increase in EAT mass, decrease in BMI and body fat mass and percentage, and no significant difference in BSA or WC with increasing %EAT. Among cardiovascular risk factors, triglyceride, γ-GT, and CRP were significantly higher, whereas HDL cholesterol level was significantly lower with increasing %EAT. No difference was observed with regard to smoking status, metabolic syndrome, and insulin resistance index. Stepwise regression analysis showed that higher %EAT was significantly associated with age, male sex, lower BMI, and higher levels of CRP, γ-GT, and triglyceride (supplementary Table S1). Moreover, %EAT was not significantly associated with insulin resistance index (supplementary Table S2).
Adiposity parameters and CAD
A total of 203 (24 %) and 54 (6.4 %) subjects were found to have obstructive CAD and severe CAD, respectively. The median calcium score was 1.0 Agatston units, although there was a very wide range (0–3682), and the score tended to increase with %EAT. Segment-stenosis score, segment-involvement score, and CAD prevalence all increased in the higher %EAT group. Coronary artery plaque was detected in 475 (56.1 %) subjects. Plaque of any type was shown in 1276 (8.8 %) segments. The most common plaque type to cause obstructive CAD or severe CAD was mixed plaque, followed by noncalcified plaque and then calcified plaque (17, 11, and 5 % for obstructive CAD, respectively, vs. 4.3, 2.1, and 0.5 % for severe CAD). Within respective composition categories, mixed plaque (59 %) was more obstructive at the 50 % threshold compared to noncalcified plaque (40 %) and calcified plaque (10 %). At the 70 % threshold, mixed plaque (8 %) and noncalcified plaque (7 %) were more often obstructive than calcified plaques (1 %). All adiposity parameters were correlated with calcium score and total plaque score (supplementary Table S2). Notably, %EAT and EAT-indexed parameters also correlated well with mixed calcified score and noncalcified plaque score. Among adiposity parameters for predicting the presence of CAD, %EAT had the highest AUC and the best discriminative performance with regard to EAT mass and other EAT-indexed parameters (Table 2). %EAT had a modest ability to detect obstructive CAD at an optimal cutoff value of 0.41 % with a sensitivity of 76 % and a specificity of 58 % and to detect severe CAD at an optimal cutoff value of 0.47 % with a sensitivity of 76 % and a specificity of 66 %. The AUCs for EAT mass and EAT-indexed parameters were not significantly different. Further, body fat mass, BMI, BSA, and WC had similar but not significant AUCs.
Prediction model
In the stepwise logistic regression model using the forward: likelihood ratio method (Table 3), calcium score, FRS, and %EAT category set by the optimal cutoff value were identified as independent predictors of obstructive or severe CAD. Subjects with %EAT >0.41 % had an increased odds ratio for obstructive CAD [3.59 (2.28–5.64), P < 0.001] after adjusting for calcium score and FRS. The classification accuracy rate was 86.1 %, which was greater than the proportion by chance accuracy criterion of 79.4 %. The stepwise regression of training samples resulted in the same number of steps as the full sample model. The classification accuracy rate for the model using the training sample was 86.6 %, compared to 85.3 % for the validation sample. Shrinkage (accuracy for 75 % training sample - accuracy for 25 % validation sample) was <2 %, implying that validation was successful. Thus, the logistic regression model based on this analysis would be effective for predicting scores in cases other than those included in the sample. Similarly, those with %EAT >0.47 % had an increased ratio for severe CAD [4.01 (2.01–7.99), P < 0.001] after adjusting for calcium score and FRS. The classification accuracy rate was 94.0 %, which was greater than the proportion by chance accuracy criterion of 88.0 %. The stepwise regression of training sample resulted in the same number of steps as the full sample model. The classification accuracy rate for the model using the training sample was 94.6 %, compared to 92.9 % for validation sample. Shrinkage was <2 %, implying the generalizability of the model to a larger population. In the final regression model, the other variables including CRP, metabolic syndrome, and categorical variables of EAT volume and indices according to BMI, BSA, or WC set by the optimal cutoff value were not included among the statistically significant predictors. Compared to AUC curves constructed by the combination of risk score plus calcium score (Table 4), adding the %EAT category marginally increased AUC by 0.01–0.02 but significantly improved CAD prediction using the IDI and NRI approaches. A slight increase in the base model AUC by calcium score and FRS was found after adding %EAT to this model but not after adding other EAT-indices. Comparing the predicted and observed CAD risk data, we found non-significant p-values for the Hosmer–Lemeshow statistics in the multiple variable models.
Subgroup analysis
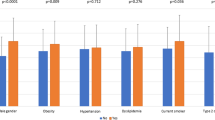
In subgroup analysis for detecting the presence of CAD, the %EAT odds ratio increased across FRS, calcium score, and obesity categories (Fig. 2). When stratifying the population by sex, we found similar results. For women, the prevalence values of obstructive CAD and severe CAD were 13.8 % (34/247) and 2.8 % (7/247), respectively. Among the adiposity parameters, %EAT had the highest AUC (0.63 for obstructive CAD, P = 0.01 vs. 0.71 for severe CAD, P < 0.05) for predicting the presence of CAD. The optimal cutoff values to detect obstructive CAD and severe CAD in women were 0.41 and 0.48 %, respectively. For men, the prevalence values of obstructive CAD and severe CAD were 28.2 % (169/599) and 7.8 % (47/599), respectively. Among adiposity parameters, %EAT had the highest AUC (0.61 for obstructive CAD, P < 0.001 vs. 0.61 for severe CAD, P = 0.01) for predicting the presence of CAD. Optimal cutoff values to detect obstructive CAD and severe CAD in men were 0.41 and 0.47 %, respectively. For the prediction model examined, we observed no significant sex interaction.
Forest plot for subgroup analysis based on categories of sex, calcium score (CaC), FRS, or body fat percentage (%BF). The %BF cutoff value for defining obesity was ≥25 % in men and ≥35 % in women. a Predicting obstructive CAD with %EAT >0.41 %. b Predicting severe CAD with %EAT >0.47 %. The odds ratios were adjusted for FRS in the calcium score category, calcium score in the Framingham risk category, or risk score and calcium score in sex or obesity categories
Discussion
To the best of our knowledge, this is the first study that reports on the relationships between epicardial fat, body fat, and CAD severity in a cohort of apparently healthy individuals without a history of coronary intervention. First, we determined that epicardial fat was disproportionately increased in older, male subjects with nonobesity, hypertriglyceridemia, fatty liver, and a low-grade inflammatory state. Second, we found that %EAT was significantly greater in patients with coronary calcification, extensive and severe coronary atherosclerosis, and obstructive plaques with noncalcified or mixed calcified components. Finally, we demonstrated that %EAT was a significant predictor of CAD; %EAT had an additional value for predicting the presence of obstructive CAD beyond the calcium score and FRS. The use of %EAT cutoff value to detect obstructive or severe CAD is valid regardless of sex, obesity, FRS, or calcium score categories.
Epicardial fat increases with body fat [6]. Increased %EAT could indicate a disproportionate increase in epicardial fat in nonobese subjects or an excessive depot of epicardial fat in obese subjects. We found both EAT mass and %EAT were associated with aging and markers of fatty liver. Moreover, despite the strong association of EAT with insulin resistance, the association was attenuated after adjusting for body fat. Generally, healthy adults have subcutaneous fat greater than 90 % of total body fat [21]. Visceral fat increased with increasing subcutaneous fat and epicardial fat shares with liver fat as a part of visceral fat. Functional subcutaneous fat stores lipids and can exert a protective modulation of vascular function by secreting adiponectin [22]. When subcutaneous adipose tissue cannot remove free fatty acids from these organs, epicardial and liver fat accumulation occurs, in conjunction with increased production of inflammatory cytokines, decreased production of adiponectin, and subsequent coronary atherosclerosis [23]. From this point of view, EAT is more like a marker of age-related increase in general adiposity with visceral fat distribution. Instead, increased %EAT is considered a manifestation of dysfunctional subcutaneous adipose tissue with direct adverse proatherogenic effects to coronary arteries.
Additionally, we found that simple anthropometric measures such as BMI, BSA, or WC might fail to illustrate the true CAD status of an individual, whereas %EAT, EAT, and its indices were successful. Moreover, %EAT was better correlated with mixed plaque score and noncalcified plaque score, whereas EAT was better correlated with mixed calcified score and calcified plaque score. The observed association between %EAT and CAD was stronger in subjects with stenosis from mixed or noncalcified plaques than in those from calcified plaques. Because mixed or noncalcified plaque was more obstructive at the 50 and 70 % thresholds than calcified plaque, these associations demonstrate that %EAT is a better predictor of CAD than is EAT. Mahabadi et al. [24] observed that EAT is associated with progression of coronary calcium burden, especially in young subjects and subjects with low calcium score at baseline. They also observed that the effect of EAT on coronary calcium progression was more pronounced in subjects with lower BMI (≤25 kg/m2) and decreases with increasing adiposity (BMI ≤25 kg/m2; P = 0.0001, BMI >40 kg/m2; P = 0.96). These findings suggest that the impact of general adiposity on the relationship between EAT and CAD is different for patients with low and high BMIs. Together with our results, these findings support the idea that EAT alone is linked to body fat-related calcified plaque, whereas a disproportionately increased EAT is further linked to more obstructive plaque components (mixed plaque and noncalcified plaque).
Our study confirmed that increased EAT mass correlates with coronary stenosis severity not only in nonobese CAD patients, but also in obese subjects. Previous studies using BSA-indexed EAT have shown similar results [25, 26]. Patients with a history of acute coronary syndrome have higher BSA-indexed EAT than those without, and an increased BSA-indexed EAT (≥50 cm3/m2) is the strongest independent determinant of the presence of total occlusion [25]. Elevated BSA-indexed EAT might be related to concurrent presence of both myocardial ischemia and ≥50 % luminal diameter stenosis in patients without known CAD [26]. We further demonstrated that %EAT is a better predictor of presence of obstructive or severe CAD than are BSA-indexed EAT and other EAT indices.
Notably, %EAT was a significant predictor of obstructive CAD and had additional value in predicting CAD beyond calcium score and FRS. %EAT >0.41 % independently predicted the presence of obstructive CAD, even after adjusting for calcium score and FRS. Additionally, subjects with %EAT >0.47 % and calcium score ≥100 or FRS ≥10 had a higher prevalence of severe CAD. These stepwise increases in %EAT, FRS, and calcium score suggest that these three independent predictors of CAD have a synergistic effect on the progression of coronary artery stenosis. This finding implies that reducing %EAT would improve CAD progression. Consistent with this speculation, peroxisome-proliferator-activated receptor γ agonists can lower %EAT by increasing subcutaneous adipose tissue [27]. The PERISCOPE study showed that pioglitazone resulted in a significant, −0.16 % decrease in coronary plaque volume over 18 months in diabetics [28]. Furthermore, recent data from animal models showed that resecting epicardial fat decreases the progression of CAD [29]. Weight loss by caloric restriction and exercise in obese subjects is associated with a decrease in EAT, but it is still unknown whether this is accompanied by a reduction in CAD progression [30].
Of note, coronary CT angiography exposes the subject to radiation, although technical advances continue to improve image quality while reducing radiation exposure. In the present study, we estimated that the radiation dose from a 64-slice scanner for prospective and retrospective ECG-gated examination was 5.3 ± 2.0 and 15.7 ± 4.4 mSv, respectively. In mid-2010 after the introduction of a 256-slice scanner with iterative reconstruction techniques [31], the corresponding doses were reduced to 3.0 ± 0.7 and 6.3 ± 1.4 mSv. Thus, the radiation dose was quite high because 55 % of the CT scanner was a conventional 64-slice scanner before mid-2010, when dose reduction techniques such as iterative reconstruction were unavailable. In fact, the radiation doses between our study and others were similar [32].
This study had several limitations. MDCT is not reliable to differentiate 50 and 70 % stenosis due to the blooming effect of the calcified components. Also, determining the severity of coronary disease by visual quantification could be arbitrary. To improve the precision of quantification, we followed the SCCT guidelines for the interpretation and reporting of coronary CT angiography [18], and the data were analyzed by an experienced radiologist. Moreover, instead of dual X-ray absorptiometry, we measured body composition with a bioimpedance analyzer, as the method is suitable for large-scale screening, and the measurements have been validated by comparison with dual X-ray absorptiometry [16, 17]. Thus, %EAT measurements had reasonable reliability and validity. Further longitudinal studies are warranted to verify the clinical applicability of %EAT in prediction of CAD risk. Participants in our study were Chinese, and further studies are needed to demonstrate whether the results are generalizable to different racial groups. For population-scale screening, studies to validate %EAT data from non-contrast CT are warranted [33].
In conclusion, in a cohort of apparently healthy subjects, a spillover of body fat at epicardium over a critical threshold is associated with a higher prevalence of obstructive or severe CAD, even after adjusting for calcium and Framingham risk scores. Therefore, instead of measuring EAT mass only, it is important to examine these subjects for both EAT mass and body fat mass and to calculate the %EAT. This screening might offer a new approach for primary prevention of CAD.
References
Mazurek T, Kiliszek M, Kobylecka M, Skubisz-Gluchowska J, Kochman J, Filipiak K, Krolicki L, Opolski G (2014) Relation of proinflammatory activity of epicardial adipose tissue to the occurrence of atrial fibrillation. Am J Cardiol 113(9):1505–1508
Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS (2008) Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 117(5):605–613
Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P (2010) Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis 210(1):150–154
Psaltis PJ, Talman AH, Munnur K, Cameron JD, Ko BS, Meredith IT, Seneviratne SK, Wong DT (2016) Relationship between epicardial fat and quantitative coronary artery plaque progression: insights from computer tomography coronary angiography. Int J Cardiovasc Imaging 32(2):317–328
Tanami Y, Jinzaki M, Kishi S, Matheson M, Vavere AL, Rochitte CE, Dewey M, Chen MY, Clouse ME, Cox C, Kuribayashi S, Lima JA, Arbab-Zadeh A (2015) Lack of association between epicardial fat volume and extent of coronary artery calcification, severity of coronary artery disease, or presence of myocardial perfusion abnormalities in a diverse, symptomatic patient population: results from the CORE320 multicenter study. Circ Cardiovasc Imaging 8(3):e002676
Silaghi A, Piercecchi-Marti MD, Grino M, Leonetti G, Alessi MC, Clement K, Dadoun F, Dutour A (2008) Epicardial adipose tissue extent: relationship with age, body fat distribution, and coronaropathy. Obesity (Silver Spring) 16(11):2424–2430
Gorter PM, de Vos AM, van der Graaf Y, Stella PR, Doevendans PA, Meijs MF, Prokop M, Visseren FL (2008) Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol 102(4):380–385
Yamamoto H, Kitagawa T, Kunita E, Tshushima H, Tatsugami F, Awai K, Kihara Y (2015) Accumulation of epicardial adipose tissue increases coronary morbidity in non-obese patients with suspected coronary artery disease. IJC Metab Endocr 8:7–12
Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, Ouyang P, Espeland MA, Lohman KK, Criqui MH, Allison M, Bluemke DA, Carr JJ (2009) The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 90(3):499–504
Mahabadi AA, Berg MH, Lehmann N, Kalsch H, Bauer M, Kara K, Dragano N, Moebus S, Jockel KH, Erbel R, Mohlenkamp S (2013) Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol 61(13):1388–1395
Gomez-Ambrosi J, Silva C, Galofre JC, Escalada J, Santos S, Millan D, Vila N, Ibanez P, Gil MJ, Valenti V, Rotellar F, Ramirez B, Salvador J, Fruhbeck G (2012) Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes (Lond) 36(2):286–294
Lahmann PH, Lissner L, Gullberg B, Berglund G (2002) A prospective study of adiposity and all-cause mortality: the Malmo Diet and Cancer Study. Obes Res 10(5):361–369
Bigaard J, Frederiksen K, Tjonneland A, Thomsen BL, Overvad K, Heitmann BL, Sorensen TI (2004) Body fat and fat-free mass and all-cause mortality. Obes Res 12(7):1042–1049
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97(18):1837–1847
Cho YG, Kang JH, Kim KA (2007) Comparison of bioelectrical impedance analysis with dual energy X-ray absorptiometry in obese women. Korean J Obes 16(2):50–57
Chien KL, Lin HJ, Lee BC, Hsu HC, Chen MF (2011) Relationship of adiposity and body composition to the status of metabolic syndrome among ethnic Chinese Taiwanese. Nutr Metab Cardiovasc Dis 21(9):643–650
Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, Nieman K, Pontone G, Raff GL (2014) SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 8(5):342–358
Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, Lippolis NJ, Berman DS, Callister TQ (2007) Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 50(12):1161–1170
Shen W, Wang Z, Punyanita M, Lei J, Sinav A, Kral JG, Imielinska C, Ross R, Heymsfield SB (2003) Adipose tissue quantification by imaging methods: a proposed classification. Obes Res 11(1):5–16
Thomas EL, Saeed N, Hajnal JV, Brynes A, Goldstone AP, Frost G, Bell JD (1998) Magnetic resonance imaging of total body fat. J Appl Physiol (1985) 85(5):1778–1785
Wildman RP, Janssen I, Khan UI, Thurston R, Barinas-Mitchell E, El Khoudary SR, Everson-Rose SA, Kazlauskaite R, Matthews KA, Sutton-Tyrrell K (2011) Subcutaneous adipose tissue in relation to subclinical atherosclerosis and cardiometabolic risk factors in midlife women. Am J Clin Nutr 93(4):719–726
Harada K, Amano T, Uetani T, Yoshida T, Kato B, Kato M, Marui N, Kumagai S, Ando H, Ishii H, Matsubara T, Murohara T (2013) Association of inflammatory markers with the morphology and extent of coronary plaque as evaluated by 64-slice multidetector computed tomography in patients with stable coronary artery disease. Int J Cardiovasc Imaging 29(5):1149–1158
Mahabadi AA, Lehmann N, Kalsch H, Robens T, Bauer M, Dykun I, Budde T, Moebus S, Jockel KH, Erbel R, Mohlenkamp S (2014) Association of epicardial adipose tissue with progression of coronary artery calcification is more pronounced in the early phase of atherosclerosis: results from the Heinz Nixdorf recall study. JACC Cardiovasc Imaging 7(9):909–916
Ueno K, Anzai T, Jinzaki M, Yamada M, Jo Y, Maekawa Y, Kawamura A, Yoshikawa T, Tanami Y, Sato K, Kuribayashi S, Ogawa S (2009) Increased epicardial fat volume quantified by 64-multidetector computed tomography is associated with coronary atherosclerosis and totally occlusive lesions. Circ J 73(10):1927–1933
Nakazato R, Dey D, Cheng VY, Gransar H, Slomka PJ, Hayes SW, Thomson LE, Friedman JD, Min JK, Berman DS (2012) Epicardial fat volume and concurrent presence of both myocardial ischemia and obstructive coronary artery disease. Atherosclerosis 221(2):422–426
Sacks HS, Fain JN, Cheema P, Bahouth SW, Garrett E, Wolf RY, Wolford D, Samaha J (2011) Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: changes associated with pioglitazone. Diabetes Care 34(3):730–733
Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A, Jure H, De Larochelliere R, Staniloae CS, Mavromatis K, Saw J, Hu B, Lincoff AM, Tuzcu EM, Investigators P (2008) Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 299(13):1561–1573
McKenney ML, Schultz KA, Boyd JH, Byrd JP, Alloosh M, Teague SD, Arce-Esquivel AA, Fain JN, Laughlin MH, Sacks HS, Sturek M (2014) Epicardial adipose excision slows the progression of porcine coronary atherosclerosis. J Cardiothorac Surg 9:2
Iacobellis G, Singh N, Wharton S, Sharma AM (2008) Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring) 16(7):1693–1697
Hou Y, Xu S, Guo W, Vembar M, Guo Q (2012) The optimal dose reduction level using iterative reconstruction with prospective ECG-triggered coronary CTA using 256-slice MDCT. Eur J Radiol 81(12):3905–3911
Raff GL (2010) Radiation dose from coronary CT angiography: five years of progress. J Cardiovasc Comput Tomogr 4(6):365–374
Ding X, Terzopoulos D, Diaz-Zamudio M, Berman DS, Slomka PJ, Dey D (2015) Automated pericardium delineation and epicardial fat volume quantification from noncontrast CT. Med Phys 42(9):5015–5026
Acknowledgments
The study was supported by grants from the National Taiwan University Hospital (NTUH.100-S1611) and Ministry of Science and Technology (MOST 104-2221-E-002-208). The authors thank the staff of the Health Management Center in National Taiwan University Hospital for their help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
B.-C. Lee and W.-J. Lee have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, BC., Lee, WJ., Lo, SC. et al. The ratio of epicardial to body fat improves the prediction of coronary artery disease beyond calcium and Framingham risk scores. Int J Cardiovasc Imaging 32 (Suppl 1), 117–127 (2016). https://doi.org/10.1007/s10554-016-0912-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-0912-2