Abstract
Right-sided heart failure is the most common cause of death in pulmonary hypertension (PH). Echocardiographic measurements of right atrial (RA) size are associated with worse outcome in PH, however the association between RA function and death in PH has not been well-described. 160 PH patients (World Health Organization groups 1–5) underwent cardiac magnetic resonance imaging (cMRI) and right heart catheterization (RHC) within 6 weeks of each other at a tertiary care academic medical center in the United States. We measured cMRI RA maximum and minimum volumes indexed to body surface area and calculated RA emptying fraction (RAEF). We evaluated the relationship between RAEF and clinical variables with death using Cox proportional hazard models. 57 deaths occurred during a median follow-up of 3.5 years (36 % died overall, 10 % per year). RAEF was directly correlated in univariate analyses with right ventricular (RV) ejection fraction, left ventricular (LV) ejection fraction, LV size, cardiac index, absence of tricuspid and pulmonic regurgitation, absence of pericardial effusion, estimated glomerular filtration rate, 6-minute walk distance, and pulmonary arterial oxygen saturation, whereas it was inversely correlated with death, BNP, heart rate, mean RA pressure, mean PA pressure, pulmonary and systemic vascular resistance, RV size, and RA size. Using multivariate analyses, RAEF had a robust inverse association with death after adjusting for measured risk factors (HR per 5 % change in RAEF: 0.83 [95 % CI 0.73–0.94], p = 0.003). In PH patients, decreased RAEF by cMRI is independently associated with worse survival after adjustment for other risk factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary hypertension (PH) is a diverse disease that is found in up to 10 % of patients undergoing routine echocardiography in a clinical setting [1]. Direct invasive hemodynamic assessment is required for confirmation of the diagnosis and initiation of PH therapy. PH-associated morbidity and mortality remain high despite available treatments including oral medications, continuous intravenous therapy, lung transplantation, and additional interventions aimed to counteract the specific causes of the various forms of PH [2–4]. Although prognostic scores have been developed for idiopathic PH (IPAH) [2, 5, 6], diagnostic and treatment algorithms for patients with PH at large remain cumbersome while the ability of individual tests to inform clinical decision-making remain limited.
There is a growing consensus that the cause of death across all five PH groups is heavily influenced by right heart failure [2]. Thus there has been a focus on right ventricular (RV) structure, function, and remodeling in PH [7–11]. Interestingly, the main clinical endpoints used in trials might generally be considered as surrogate markers of right heart function and reserve: World Health Organization (WHO) functional classification, 6-minute walk distance (6MWD), exercise testing, cardiac biomarkers, and anatomic/functional echocardiographic parameters [5, 12–19]. Echocardiographic studies have demonstrated the prognostic value in IPAH of tricuspid annular plane systolic excursion (TAPSE), RV myocardial performance index, right atrial (RA) size, and pericardial effusion [20–24]. Although echocardiography is the mainstay of evaluating the right heart in routine clinical practice, cardiac magnetic resonance imaging (cMRI) is considered the gold standard of RV imaging; it allows for a reproducible and accurate evaluation of the structural and functional characteristics of this chamber that can otherwise be challenging to image [25, 26]. Stroke volume index, RV ejection fraction (RVEF), and indexed RV end-diastolic and end-systolic volumes, as assessed by cMRI, have been shown previously as important non-invasive prognostic markers in IPAH [27–31]. There has even been recent work examining the added benefit of RA functional and structural measurements in prognostic scores for IPAH patients [6, 32, 33]. In the absence of primary abnormalities of the tricuspid valve, RA enlargement is generally viewed as a manifestation of high RA pressure due to functional tricuspid regurgitation (TR) or elevated RV diastolic pressure, both consequences of RV failure. However, the association between RA structure and function and RV failure and death in all types of PH has not been well-described. Moreover, prognostic classification for idiopathic IPAH patients remains imperfect [2, 5, 6] and prognostic classification for other types of PH patients is even less well defined. The purpose of our study is to evaluate the association of RA structure and function with RVEF as well as with time to death in PH patients. We assessed RA and RV parameters by cMRI given its uniquely accurate and reproducible ability to view these chambers. We hypothesized that cMRI-determined RA emptying fraction (RAEF) is directly associated with RVEF and is inversely associated with death independently from RVEF (and other commonly assessed risk factors) in patients with PH.
Materials and methods
Study population, inclusion criteria, and assessed variables
Data were retrospectively collected on a cohort of 172 consecutive patients initially seen at the University of Texas Southwestern Medical Center (Dallas, TX) between 2008 and 2010 with a diagnosis of PH, including WHO groups I–V. We excluded six patients who did not have a cMRI and right heart catheterization (RHC) performed within 6 weeks of each other around the time of the index clinic visit. We excluded another six patients whose mean pulmonary arterial pressure was not ≥25 mmHg by RHC. Classification of PH type was made according to the clinical classification scheme of the Fifth World Symposium (Nice, France, 2013) [34]. In the evaluation for PH subtype, data closest to the RHC date was collected from chart review, including pulmonary function testing (PFT) and 6MWD. The diagnosis of PH was determined by two independent physicians (KD and KMC).
Patient charts were reviewed for the following variables (closest to but within 6 months of the RHC): age, sex, height, weight, ethnicity, comorbid medical conditions, medications, WHO PH etiology, WHO functional class, BNP level, troponin, estimated glomerular filtration rate (eGFR by the Modified Diet in Renal Disease equation), hemoglobin, heart rate (during cMRI), 6MWD, PFT results (single breath carbon monoxide diffusing capacity = DLCOc, forced vital capacity = FVC, forced expiratory volume in one second = FEV1), hemodynamic measurements (mean arterial pressure = MAP, systemic arterial oxygen saturation, mean RA pressure = mRAP, mean pulmonary arterial pressures = mPAP, PA oxygen saturation, mean pulmonary capillary wedge pressure = PCWP, Fick cardiac index = CI, Fick systemic vascular resistance = SVR, and Fick pulmonary vascular resistance = PVR), echocardiographic measurements (graded tricuspid regurgitation and graded pulmonic regurgitation), and presence of pericardial effusion (graded either by echocardiogram or cMRI). We analyzed cMRI studies performed within 6 weeks of RHC for the following: indexed RV end-diastolic volume = RVEDVOI, RVEF, indexed left ventricular end-diastolic volume = LVEDVOI, left ventricular ejection fraction = LVEF, and indexed left ventricular mass = LVMASSI, indexed RA end-diastolic and end-systolic volumes = RAEDVOI and RAESVOI, RA ejection fraction = RAEF. Survival and organ transplantation were determined by chart review upon study completion.
Imaging procedures
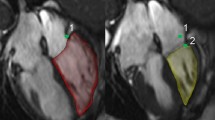
All cMRIs were performed on a GE 1.5-T scanner (GE Healthcare, Milwaukee, Wisconsin) using steady-state free precession cine short-axis images. Cardiac volume and mass measurements were performed using MASS software (MEDIS, Leiden, Netherlands) with prospective ECG gating on the acquisition sequence. RA areas were measured from the apical four chamber view only, by tracing the endocardial contour at the time of maximum distension [RA end-diastolic (ED) area], which occurs at LV end-systole, and at the time of smallest RA area [RA end-systolic (ES) area]. Measurements were made in accordance with published recommendations on four chamber measurements of RA area [35]. RA length was measured from the midpoint of the tricuspid annular plane to the back wall of the right atrium (Fig. 1). To approximate volumes, the single plane area-length method was used. RAEF was calculated as the difference between cMRI derived RA maximum volume (RAmax) and RA minimum volume (RAmin), divided by RAmax. All volumes were indexed to BSA. Only four chamber views that excluded the LV outflow tract throughout the cardiac cycle were used for analysis. For quality control, all measurements of RA parameters were performed by a single physician (KD) after satisfactory completion of a training set of images (within 10 % of measured training set values). Contours were redrawn by a second physician (SM) on 5 % of randomly selected subjects to assess for intra- and inter-observer variability in the newly acquired cMRI measures.
In order to define gender-specific thresholds for normal RAEF, RA measurements were performed in a previously described healthy reference subpopulation of the Dallas Heart Study that underwent cMRI [36]. Low RAEF and low RAEDVOl were defined as RAEF or RAEDVOI less than the fifth percentile of gender-specific values respectively in this healthy normal population.
Clinical outcomes
Patients were followed from the date of initial clinical examination until death or the end of follow-up on January 31, 2014. Death of a participant was determined using the National Death Index and was corroborated with the patient’s medical record. All-cause mortality was used as the primary outcome variable.
Statistical analysis
Statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA). Continuous data are presented as median plus interquartile range (25th and 75th percentiles), and categorical data are presented as percentages with significance determined by the Jonckheere–Terpstra trend test. Intra- and inter-observer reproducibility was determined through the use of intraclass correlation coefficients (ICC) by mixed modeling. Multivariable Cox regression analyses were generated to test the association between death and RAEF (continuous and tertiles). Models were adjusted for the described variables. Assumptions for the Cox regression models were satisfied through the use of Schoenfeld residuals. All reported p values are two-sided with p < 0.05 considered statistically significant.
Results
Validation of intra- and inter-observer reproducibility (as described in the “Materials and methods” section) showed that our analyses of RAEF and RAEDVOI were highly reproducible. Intra-observer ICC for our primary reader was 0.99 and 0.97 for RAEDVOI and RAEF, respectively. Inter-observer ICC between our primary and secondary readers was 0.97 and 0.90 for RAEDVOI and RAEF, respectively.
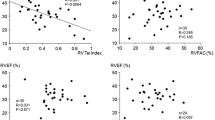
Of the 160 total outpatients that we studied, 83 were incident and 57 died during a median follow-up of 3.5 years. Patients were predominantly white, female, and middle-aged (Table 1). WHO group 1 PH was the most prevalent type of PH within each tertile with an idiopathic etiology being the most common WHO group 1 subtype for each tertile. RAEF tertile associated most strongly with measures of RV structure and function that included strong direct associations with cardiac index, PA oxygen saturation, and RVEF, and strong inverse associations with mean RA pressure, RAEDVOI, RAESVOI, RVEDVOI and BNP (Tables 1, 2).
Univariate analyses of survival compared with cardiac MRI measurements listed within Table 2 demonstrated significantly improved survival with higher RAEF (p < 0.0001, HR 0.83 per 5 % increase in RAEF, CI [0.77, 0.90]) or RVEF (p = 0.001, HR 0.86 per 5 % increase in RVEF, CI [0.78, 0.94]). RAEF itself was directly associated with RVEF by univariate analysis (ρ = 0.39, p < 0.001).
Kaplan–Meier analysis demonstrated a significant association between RAEF tertile and patient mortality (p < 0.0001) with a stepwise increase in mortality moving from high RAEF to moderate RAEF to low RAEF (Fig. 2, p < 0.05 for each pairwise comparison). There was no significant association between RAEDVOI tertile and patient mortality (p = 0.46) by Kaplan–Meier estimation (Fig. 3). A Kaplan–Meier plot assessing all four combinations of RAEF (normal vs. low, cutpoint ≤31.1 %/23.4 % based on fifth percentile for women/men of sex-specific healthy normal values in the Dallas Heart Study) and RAEDVOI (normal vs. high, cutpoint ≥49.0/57.8 mL based on 95th percentile for women/men of sex-specific healthy normal values in the Dallas Heart Study) demonstrates that RAEF, rather than RAEDVOI, drives the association with mortality in these PH patients (Fig. 4, p = 0.001 overall and p < 0.05 for all but two pairwise comparisons: normal RAEF/normal RAEDVOI vs. normal RAEF/high RAEDVOI and low RAEF/normal RAEDVOI vs. low RAEF/high RAEDVOI).
Kaplan–Meier survival curves showing the relationship between RAEF and RAEDVOI. Lower RAEF correlated with higher mortality, but RAEDVOI did not correlate with a change in mortality. Each pairwise comparison was statistically significant (p < 0.05) except as marked (N.S.). RAEF right atrial ejection fraction, RAEDVOI right atrial end-diastolic volume index
RAEF was robustly associated with overall survival in several multivariate analyses (Fig. 5). The inverse relationship between RAEF tertile (tertile 3 vs. tertiles 1 or 2 as shown for each of these statistical models) and overall survival remained significant for all of the following multivariate adjustments: (1) age and sex; (2) age, sex, BNP, 6MWD, and LVEF; (3) age, sex, and 6MWD; (4) age, sex, RVEDVOI, RVEF, LVEDVOI, LVEF, RAEDVOI, and RAESVOI; (5) age, sex, heart rate, CI, SVR, PVR, pulmonary oxygen saturation, mRAP, and mPAP; (6) age, sex, BNP, and eGFR; (7) age, sex, pericardial effusion, tricuspid regurgitation, and pulmonic regurgitation; (8) Complex Model #1 age, sex, BNP, pericardial effusion, mRAP, mPAP, PVR, LVEF, RVEF, RVEDVOI, and RAEDVOI; and (9) Complex Model #2 age, sex, and all variables (other than RAEF) that reached statistical significance in Table 2. In multivariate model 8 above (= Complex Model #1), the hazard ratio for survival per 5 % increase in RAEF = 0.83 (95 % CI 0.73–0.94, p = 0.003).
Multivariate analyses of survival by RAEF tertile after correction for the listed measurements. Forest plot of multiple statistical models that each compare tertile 3 RAEF mortality vs. either tertile 1 or 2 mortality as indicated following adjustment for the listed measurements. The results cumulatively show that RAEF has a robust association with mortality despite adjustment for multiple other measured variables. Sig = statistically significant (p < 0.05) between RAEF tertiles as listed in Table 2. Complex Model 1 = age, sex, BNP, pericardial effusion, mean right atrial pressure, mean pulmonary arterial pressure, Fick pulmonary vascular resistance, left ventricular ejection fraction, right ventricular ejection fraction, right ventricular end-diastolic volume index, and right atrial end-diastolic volume index. Complex Model 2 = age, sex, and all variables (other than RAEF) that reached statistical significance (p < 0.05) in Table 2. RAEF right atrial ejection fraction
Discussion
Direct visualization of pulmonary vascular changes in PH patients is not possible antemortem, but a growing consensus links the ultimate cause of death in PH patients to closely related right heart structure and function. Our results extend this association using a high-fidelity imaging technique (cMRI) for the right side of the heart, and we show in this retrospective cohort analysis that RAEF has a robust inverse correlation with the likelihood of mortality even after adjusting for other measured risk factors. It is notable to show that RAEF serves as an independent risk factor for death since (1) the predominant focus on the right heart in PH has been on the RV rather than the RA and (2) RAEF varies even amongst those with preserved RVEF. Although speculative, it is possible that the latter finding may reflect a sensitivity of RA function to both RV diastolic and RV systolic function. This study is also notable in that it included patients with all types of PH rather than just IPAH.
Our patient population was a fairly typical one for PH in that it was predominantly composed of white, middle-aged females with WHO group 1 etiology. They had a mortality in the expected range ~10 % per year and received standard contemporary treatments for PH. Their RA function varied considerably from a median RAEF of 11 % in the lowest tertile to 47 % in the highest tertile, and we showed that RAEF independently associated with patient survival even though it was separately associated with RVEF as well. Indexed RAEDV, by contrast, was associated with survival in this study only in univariate analyses but not using Kaplan–Meier or multivariate analyses. This is in contrast to a recent report from Haddad et al. where they found RAEDV more useful than RAEF in deriving a prognostic echocardiographic ‘right heart score’ for IPAH patients [6]. While the difference in results between our studies may possibly be explained by the different patient populations or different imaging modalities used (cMRI may more accurately determine the RAEF, for example, and Haddad et al. did not use cMRI for their derivation cohort of patients), this should not distract from the important general conclusion that RA structure and function are important predictors for hard patient outcomes in patients with PH. In fact, subsequent work from the same group has further corroborated our results by showing that in IPAH (as opposed to general PH in our study) there is a clear link between RAEF and patient mortality [33]. Their point estimated hazard ratio of low vs. high echo-determined total RAEF on total mortality was 4.2, which compares favorably with ours at 5.0 (comparing the lowest and highest RAEF tertiles in this study by cMRI without adjusting for other measured factors). We thus have independently established a similar prognostic association between RAEF and mortality in patients with PH using complementary imaging modalities. This at once helps confirm the results and further demonstrates the robustness of this association.
RAEF was associated with RVEF, and each was also associated with survival in univariate analyses. In fact, the point estimated survival HR’s per 5 % increase in RAEF and RVEF were nearly identical at 0.83 and 0.86, respectively. When considering that multivariate analyses additionally showed that RAEF has a similar HR for death even after correcting for multiple variables (including RVEF; see in particular Complex Model 1 as described above), it is clear that RAEF seems to serve as an independent prognostic factor for hard patient outcomes in PH. This does not negate the importance of the RV in PH but merely suggests that the RA has a particularly important prognostic role in this disease process.
There are several limitations of this study. The modest size (160 patients after application of exclusion criteria) does not allow for additional post-hoc subgroup analyses, and the general study design (retrospective cohort) has known limitations at establishing an etiologic link between variables such as RAEF and survival. Our patient demographics, while typical of this problem in the United States, may not allow universal extrapolation of our findings. RA volume assessment by Simpson’s method of discs would have been a more accurate assessment of RA volume than the calculated single plane area-length measurements used [35]. Our retrospective review of clinically acquired studies prohibited direct volume measurements as the short-axis images of the heart were truncated just basal to the atrioventricular valves and therefore did not cover the entire right atrium. However, the correlation between the area-length measurement and the volumetric measurement of RA volume is relatively good both by echocardiography (r = 0.70 for area-length method vs. 3D echo method [37]) and MRI (r = 0.65 vs. real RA volume [35]). While we assessed RAEF by cMRI, which is not available for patient care in all areas, this parameter can also be estimated by echocardiography as well such that further analyses of echo-derived RAEF in PH will be of interest. Nevertheless, we believe that our findings help support the growing recognition that right heart (RA and RV) structure and function serve important prognostic roles for patients with PH.
The fact that RAEF was associated with overall cardiac function (heart rate, LVEF, RVEF, cardiac index, SVR), PA parameters (oxygen saturation, PA pressures, PVR), serum-derived measurements (BNP, eGFR), 6MWD, and echocardiographic parameters (pericardial effusion, tricuspid regurgitation, pulmonic regurgitation) is intriguing but does not offer specific insight into the etiology of these associations. Future investigation to delineate these complex relationships as well as to evaluate whether serial assessment of RA and RV parameters can offer prognostic reclassification of patients following initiation of PH therapyis needed.
Perspectives
There is a growing appreciation for the importance of the right side of the heart in the prognosis for patients with PH. Both RA size and function have been independently linked to mortality in such individuals. Whether these parameters simply serve as sensitive markers of right heart systolic and diastolic function or whether these parameters are more heavily influenced by other variables remains to be determined. Likewise, it remains to be seen whether serial assessment of these parameters may aid in prognostic reclassification after initiation or change in therapy for PH.
References
Strange G, Playford D, Stewart S, Deague JA, Nelson H, Kent A, Gabbay E (2012) Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart 98(24):1805–1811. doi:10.1136/heartjnl-2012-301992
D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, William GW, Wu M (1991) Survival in patients with primary pulmonary hypertension. Ann Intern Med 115(5):343–349. doi:10.7326/0003-4819-115-5-343
Badesch DB, McLaughlin VV, Delcroix M, Dario Vizza C, Olschewski H, Sitbon O, Barst RJ (2004) Prostanoid therapy for pulmonary arterial hypertension. J Am Coll Cardiol 12(Suppl S):56S–61S. doi:10.1016/j.jacc.2004.02.036
Keogh AM, Mayer E, Benza RL, Corris P, Dartevelle PG, Frost AE, Kim NH, Lang IM, Pepke-Zaba J, Sandoval J (2009) Interventional and surgical modalities of treatment in pulmonary hypertension. J Am Coll Cardiol 54(1 Suppl):S67–S77. doi:10.1016/j.jacc.2009.04.016
Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost AE, Barst RJ, Badesch DB, Elliot CG, Liou TG, McGoon MD (2010) Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 122:164–172. doi:10.1161/CIRCULATIONAHA.109.898122
Haddad F, Spruijt OA, Denault AY, Mercier O, Brunner N, Furman D, Fadel E, Bogaard HJ, Schnittger I, Vrtovec B, Wu JC, de Jesus Perez V, Vonk-Noordegraaf A, Zamanian RT (2015) Right heart score for predicting outcome in idiopathic, familial, or drug- and toxin-associated pulmonary arterial hypertension. J Am Coll Cardiol Cardiovasc Imaging 8(6):627–638. doi:10.1016/j.jcmg.2014.12.029
Haddad F, Doyle R, Murphy DJ, Hunt SA (2008) Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation 117(13):1717–1731. doi:10.1161/CIRCULATIONAHA.107.653584
Haddad F, Hunt SA, Rosenthal DN, Murphy DJ (2008) Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 117(11):1436–1448. doi:10.1161/CIRCULATIONAHA.107.653576
Champion HC, Michelakis ED, Hassoun PM (2009) Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation 120(11):992–1007. doi:10.1161/CIRCULATIONAHA.106.674028
Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF (2009) The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 135(3):794–804. doi:10.1378/chest.08-0492
Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB, National Heart Lung and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure (2006) Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Circulation 114(17):1883–1891. doi:10.1161/CIRCULATIONAHA.106.632208
Filusch A, Giannitsis E, Katus HA, Meyer FJ (2010) High-sensitivity troponin T: a novel biomarker for prognosis and disease severity in patients with pulmonary arterial hypertension. Clin Sci 119:207–213. DOI:10.1042/CS20100014
Velez-Martinez M, Ayers C, Mishkin JD, Bartolome SB, Garcia CK, Torres F, Drazner MH, de Lemos JA, Turer AT, Chin KM (2013) Association of cardiac troponin I with disease severity and outcomes in patients with pulmonary hypertension. Am J Cardiol 111(12):1812–1817. doi:10.1016/j.amjcard.2013.02.036
Forfia P, Mathai S, Fisher M, Houston-Harris T, Hemnes AR, Champion HC, Girgis RE, Hassoun PM (2008) Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 177:1364–1369. doi:10.1164/rccm.200712-1876OC
Heresi GA, Tang WHW, Aytekin M, Hammel J, Hazen SL, Dweik RA (2011) Sensitive cardiac troponin I predicts poor outcomes in pulmonary arterial hypertension. Eur Respir J 39:939–944. doi:10.1183/09031936.00067011
Miyamoto S, Nagaya N, Satoh T, Toru S, Kyotani S, Sakamaki F, Fujita M, Nakanishi N, Miyatake K (2000) Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 161:487–492. doi:10.1164/ajrccm.161.2.9906015
Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, Kakishita M, Fujushima K, Okano Y, Nakanishi N, Miyatake K, Kangawa K (2000) Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation 102:865–870. doi:10.1161/01.CIR.102.8.865
Provencher S, Herve P, Sitbon O, Humbert M, Simonneau G, Chemla D (2008) Changes in exercise haemodynamics during treatment in pulmonary arterial hypertension. Eur Respir J 32(2):393–398. doi:10.1183/09031936.00009008
Shah SJ, Thenappan T, Rich S, Tian L, Archer SL, Gomberg-Maitland M (2008) Association of serum creatinine with abnormal hemodynamics and mortality in pulmonary arterial hypertension. Circulation 117:2475–2483. doi:10.1161/CIRCULATIONAHA.107.719500
Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PM (2006) Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 174:1034–1041. doi:10.1164/rccm.200604-547OC
Ghio S, Klersy C, Magrini G, D’Armini AM, Seelsi L, Raineri C, Pasotti M, Serio A, Campana C, Vigano M (2010) Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol 140:272–278. doi:10.1016/j.ijcard.2008.11.051
Ghio S, Pazzano AS, Klersy C, Scelsi L, Raineri C, Camporotondo R, D’Armini A, Visconti LO (2011) Clinical and prognostic relevance of echocardiographic assessment of right ventricular geometry in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol 107:628–632. doi:10.1016/j.amjcard.2010.10.027
Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao J-F, Maalouf JF, Ammash NM, McCully RB, Miller FA, Pellikka PA, Oh JK, Kane GC (2011) Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest 139:1299–1309. doi:10.1378/chest.10-2015
Vonk MC, Sander MH, van den Hoogen FHJ, van Riel PLCM, Verheugt FWA, van Dijk APJ (2007) Right ventricle Tei-Index: a tool to increase the accuracy of non-invasive detection of pulmonary arterial hypertension in connective diseases. Eur J Echocardiogr 8:317–321. doi:10.1016/j.euje.2006.06.002
Dell’Italia LJ (2012) Anatomy and physiology of the right ventricle. Cardiol Clin 30(2):167–187. doi:10.1016/j.ccl.2012.03.009
Sanz J, Conroy J, Narula J (2012) Imaging of the right ventricle. Cardiol Clin 30:189–203. doi:10.1016/j.ccl.2012.03.001
van Wolferen SA, Johannes MT, Boonstra A, Marques KMJ, Bronzwaer JGF, Spreeuwenberg MD, Postmus PE, Vonk-Noordegraf A (2007) Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 28:1250–1257. doi:10.1093/eurheartj/ehl477
Hagger D, Condliffe R, Woodhouse N, Elliot CA, Armstrong IJ, Davies C, Hill C, Akil M, Wild JM, Kiely DG (2009) Ventricular mass index correlates with pulmonary artery pressure and predicts survival in suspected systemic sclerosis-associated pulmonary arterial hypertension. Rheumatology 48:1137–1142. doi:10.1093/rheumatology/kep187
van de Veerdonk MC, Kind T, Marcus JT, Mauritz G-J, Heymans MW, Bogaard H-J, Boonstra A, Marques KMJ, Westerhof N, Vonk-Noordegraf A (2011) Progressive right ventricuular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 58(24):2511–2519. doi:10.1016/j.jacc.2011.06.068
Vonk Noordegraaf A, Gallè N (2011) The role of the right ventricle in pulmonary arterial hypertension. Eur Respir Rev 20(122):243–253. doi:10.1183/09059180.00006511
Mauritz G-J, Kind T, Marcus JT, Bogaard HJ, van de Veerdonk MC, Postmus PE, Boonstra A, Westerhof N, vonk Noordegraaf A (2012) Progressive changes in right ventricular geometric shortening and long-term survival in pulmonary arterial hypertension. Chest 141(4):935–943. doi:10.1378/chest.10-3277
Raymond RJ, Hinkerliter AL, Willis PW, Ralph D, Caldwell EJ, Willliam W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, Schwartz T, Koch G, Clayton LM, Jobsis MM, Crow JW, Long W (2002) Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 24(7):1214–1219. doi:10.1016/S0735-1097(02)01744-8
Brunner NW, Haddad F, Kobayashi Y, Hsi A, Swiston JR, Gin KG, Zamanian RT (2015) Prognostic utility of right atrial emptying fractions in pulmonary arterial hypertension. Pulm Circulation 5(3):473–480. doi:10.1086/682218
Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R (2013) Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62 (25 Suppl):D34–D41. doi:10.1016/j.jacc.2013.10.029
Maceira AM, Cosin-Sales J, Roughton M, Prasad SK, Pennell DJ (2013) Reference right atrial dimensions and volume estimation by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 15:29. doi:10.1186/1532-429X-15-29
Chung AK, Das SR, Leonard D, Peshock RM, Kazi F, Abdullah SM, Canham RM, Levine BD, Drazner MH (2006) Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the Dallas Heart Study. Circulation 113(12):1597–1604. doi:10.1161/CIRCULATIONAHA.105.574400
Muller H, Burri H, Lerch R (2008) Evaluation of right atrial size in patients with atrial arrhythmias: comparison of 2D versus real time 3D echocardiography. Echocardiography 25(6):617–623. doi:10.1111/j.1540-8175.2008.00674.x
Author contribution
All authors contributed intellectually to this article. M.D., K.D., and S.M. primarily interpreted data and wrote the article. K.D., W.C., and S.M. contributed most to data collection. K.D. and C.A. contributed most to statistical analysis. K.C., F.T., and S.M. contributed most to initial study design. All authors have approved the final article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.D., K.D., W.C.: None. K.C.: Research grants from Actelion, Bayer, Geon, Gilead, GlaxoSmithKline, NIH, Novartis, United Therapeutics; honoraria from Actelion, Bayer, Gilead. F.T.: Research grants from Akaria, Bayer, Geon, Gilead, Medtronic; consultant/advisory board with Actelion, Bayer, Gilead, LungLLC, Novartis, United Therapeutics. S.M.: Research grants from ACC/GE Healthcare Career Development Award, National Center for Advancing Translational Sciences, NIH, UT-STAR.
Ethical approval
All procedures involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of retrospective study formal consent is not required.
Additional information
Konstadina Darsaklis and Matthew E. Dickson have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Darsaklis, K., Dickson, M.E., Cornwell, W. et al. Right atrial emptying fraction non-invasively predicts mortality in pulmonary hypertension. Int J Cardiovasc Imaging 32, 1121–1130 (2016). https://doi.org/10.1007/s10554-016-0883-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-0883-3