Abstract
The aim of this study was to determine the correlation between the fractional flow reserve (FFR) values and volumetric intravascular ultrasound (IVUS) parameters derived from classic gray-scale IVUS and integrated backscatter (IB)-IVUS, taking into account known confounding factors. Patients with unstable angina pectoris with the frequent development of vulnerable plaques often showed the discrepancy between the FFR value and the quantitative coronary angiography findings. Our target population was 107 consecutive subjects with 114 isolated lesions who were scheduled for elective coronary angiography. The FFR was calculated as the mean distal coronary pressure divided by the mean aortic pressure during maximal hyperemia. Various volumetric parameters such as lipid plaque volume (LPV) and percentage of LPV (%LPV) were measured using IB-IVUS. Simple and multivariate linear regression analysis was employed to evaluate the correlation between FFR values and various classic gray-scale IVUS and IB-IVUS parameters. The Akaike information criterion (AIC) was used to compare the goodness of fit in an each model. Both the %LPV (r = −0.24; p = 0.01) and LPV (r = −0.40; p < 0.01) were significantly correlated with the FFR value. Only the LPV (AIC = −147.0; p = 0.006) and %LPV (AIC = −152.9; p = 0.005) proved to be independent predictors for the FFR value even after the adjustment of known confounding factors. The volumetric assessment by IB-IVUS could provide better information in terms of the relationship between plaque morphology and the FFR values as compared to the classic IVUS 2-dimensional gray-scale analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The potential benefit of revascularization depends on the presence of myocardial ischemia. Therefore, the careful identification of ischemia-inducing stenosis allows for a greater benefit from revascularization, especially in patients with stable angina pectoris (SAP) [1–6]. Intravascular ultrasound (IVUS) and fractional flow reserve (FFR) are frequently used for the assessment of relevant coronary artery stenosis [7]. Previous studies primarily focused on a 2-dimensional analysis have shown a relatively good correlation between FFR and IVUS parameters [8]. However, the functional significance of a coronary stenosis is determined by both the severity of stenosis and amount of myocardium supplied, thereby necessitating a longitudinal assessment such as a 3-dimensional lesion analysis. The recently-developed integrated backscatter (IB-IVUS) allows the volumetric analysis of tissue components of coronary plaques in vivo [9, 10]. Therefore, we performed this study to determine the correlation between the FFR values and IVUS parameters derived from IB-IVUS as well as classic gray-scale IVUS, taking into account known confounding factors.
Methods
Patients and study design
This prospectively planned observational study was designed to evaluate the correlation between FFR values and volumetric plaque characteristics in the isolated culprit lesion using IB-IVUS. Between June 2011 and May 2013, our target population was 114 consecutive subjects with 123 isolated lesions who were scheduled for elective coronary angiography (CAG). The exclusion criteria were acute coronary syndrome indicating unstable angina pectoris (n = 3), lesions related to old myocardial infarction (n = 3) or prior coronary intervention (n = 1), and lesions with poor IVUS images (n = 2). Following the exclusion of patients, a total of 107 patients with 114 lesions were available for the evaluation. This study was approved by the institutional review board at Aichi Medical University; all the patients provided written informed consent, and the study complied with the principles of the Declaration of Helsinki.
CAG and FFR measurement
Before CAG was performed, an intracoronary injection of isosorbide dinitrate (0.5 mg) was administered to prevent coronary spasm. For quantitative coronary angiography (QCA), the reference diameter and percent diameter stenosis were measured by a validated automated edge-detection program (CMS;-MEDIS Medical Imaging Systems, Leiden, The Netherlands). FFR was calculated as previously described [4–6]. In brief, equalization was performed with the guide wire sensor positioned at the guiding catheter tip. The 0.014-in. pressure guide-wire (St. Jude Medical, Minneapolis, MN, USA) was then advanced distally to the stenosis, and FFR was measured at maximal hyperemia induced by intravenous adenosine triphosphate administered at 150 μ/kg/min through a central or forearm vein. The FFR was subsequently calculated as the mean distal coronary pressure divided by the mean aortic pressure during maximal hyperemia. Functional significance was defined as FFR values of ≤0.80 [11]. The present study measured FFR in the isolated lesions with intermediate to severe stenosis (26–90 % of the vessel diameter). FFR measurements were deferred when vessels had no significant stenosis (≤25 %) as observed on CAG.
Measurements of classic gray-scale IVUS and IB-IVUS parameters
We assessed coronary plaque characteristics using IB-IVUS. IVUS catheters (40 MHz) were inserted as distally as possible into the target vessels. Continuous ultrasound imaging was performed during withdrawal of the catheter at a constant rate of 0.5 mm/s. Classic IVUS and IB-IVUS parameters were measured at the isolated lesions with a plaque burden ≥40 % of the three coronary vessels. The mean length of the analyzed segment was 26.0 mm (52 IB-IVUS images) per patient. 2- and 3-dimensional analyses of classic IVUS images were performed for vessel, lumen, and plaque area (PA), and vessel, lumen, and plaque volume (PV) (the sum of each cross-sectional area of 1-mm axial intervals for the analyzed segments). 2- and 3-dimensional analyses of IB-IVUS were performed for fibrous plaque area (FPA), lipid plaque area (LPA), fibrous plaque volume (FPV), and lipid plaque volume (LPV). A personal computer (Windows XP Professional, CPU: 3.4 GHz) equipped with commercially available custom software (VISIWAVE IB, Terumo Co, Japan) was connected to the IVUS imaging system (VISIWAVE, Terumo Co) to obtain radio frequencies and signal trigger outputs. Ultrasound backscattered signals were acquired using a 40-MHz mechanically rotating IVUS catheter (motorized pullback 0.5 mm/s), digitized, and subjected to spectral analysis. IB values for each tissue component were calculated as average power levels (measured in decibels) using a fast Fourier transform of the frequency component of backscattered signals from a small tissue volume [12, 13]. The percentage of fibrous area (FPA/PA) and the percentage of lipid plaque area (LPA/PA) were automatically calculated by IB-IVUS system. Then FPV and LPV (sum of FPA and LPA in each CSA at 1 mm axis interval for the average of 26 IB-IVUS images per patient, respectively) were calculated. The percentage of fibrous plaque volume (FPV/PV, %FPV) and lipid plaque volume (LPV/PV, %LPV) were also calculated. The segmentation of each tissue component was entirely automated [14]; an excellent correlation between IB-IVUS and histology has been previously reported in validation studies [13, 15]. IVUS measurements were conducted independently by 2 physicians (SS and HT) blinded to the patients’ clinical characteristics.
Definition of risk factors
Hypertension was defined as a history or presence of hypertension with systolic blood pressure of >140 mmHg and/or diastolic blood pressure of >90 mmHg, or anti-hypertensive treatment. Diabetes mellitus was defined as a history of diabetes mellitus, a fasting plasma glucose concentration of >126 mg/dL, and/or anti-hyperglycemic treatment. Smoking status was defined as current or cessation within 6 months before CAG. Dyslipidemia was defined as a history or presence of dyslipidemia, low-density lipoprotein-cholesterol level of >140 mg/dl and/or high-density lipoprotein-cholesterol level of <40 mmHg and/or triglyceride of >150 mg/dl, or treatment with any lipid lowering agents.
Statistical analyses
Continuous and categorical variables are expressed as the mean ± SD and proportions, respectively. Simple linear regression analysis was employed to evaluate the correlation between FFR values and various classic gray-scale and IB-IVUS parameters. Multivariable analysis was performed to identify independent correlates of FFR values with linear regression. On the multivariate linear regression analyses, the Akaike information criterion was used to compare the goodness of fit between the five models [16, 17]. A p value of <0.05 was considered statistically significant.
Results
Baseline clinical and angiographic findings
Table 1 outlines the baseline clinical and angiographic findings of the 107 patients included in the study. The mean age was 68 ± 9 years; 74 and 56 % of the patients were male and diabetics, respectively. In the QCA analysis, the mean reference diameter and lesion length were 2.6 ± 0.6 and 20.6 ± 11.9 mm, respectively. The mean FFR value was 0.70 ± 0.11, and 85.1 % of the patients had lesions with FFR values of ≤0.80.
Association between FFR values and the CAG and IVUS findings
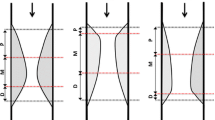
Table 2 shows the findings for the classic IVUS and IB-IVUS parameters and the results of the simple linear regression between these parameters and the FFR value. The lesion length measured by IVUS was significantly correlated with the FFR value (r = −0.40; p < 0.01). For the 2-dimensional IVUS analysis, no correlation was observed between the classis IVUS or IB-IVUS parameters and the FFR value (Fig. 1a). Meanwhile, for the 3-dimensional analysis, both the PV (r = −0.34; p < 0.01) and LPV (r = −0.40; p < 0.01) were significantly correlated with the FFR value (Fig. 1b). Figure 2 shows the relationship between the classic IVUS and IB-IVUS parameters with the threshold of FFR values defined as ≤0.80. Lesions with significant functional ischemia had greater PV (186 ± 100 vs. 104 ± 64 mm3), LPV (91 ± 59 vs. 48 ± 45 mm3), and FPV (90 ± 50 vs. 56 ± 41 mm3) compared with lesions without functional ischemia. In the multivariate linear regression model with FFR values as shown in Table 3, the lesion length as measured by IVUS was significantly and independently correlated with the FFR value (Model 1), consistent with previous reports. The PV (mm3) and %PV (%) as measured by the 3-dimensional classic gray-scale IVUS were not independently correlated with the FFR value (Models 2 and 3). Meanwhile, the LPV (mm3) and %LPV (%) as measured by 3-dimensional IB-IVUS proved to be independent predictors for the FFR value even after adjustment of confounding factors (Models 4 and 5). When the FPV (mm3) was included in the model as an independent factor, the LPV (mm3) remained to be a significant and independent predictor for the FFR value (Model 6). Figure 3 shows representative images of a lesion with functional ischemia and large LPV in (a), and a lesion without functional ischemia and small LPV in (b). The PV, LPV, FPV, lumen volume, and FFR were 186.6, 119.1, 66.2, 148.2, and 0.68 mm3, respectively, in (a), and 144.9, 43.9, 96.6, 139.7, and 0.81 mm3, respectively, in (b).
a Correlation between FFR and 2-dimensional classic and IB-IVUS parameters. There were no significant correlation between FFR and 2-dimensional classic and IB-IVUS parameters. Each parameter was measured at the minimum luminal area. FFR fractional flow reserve, IB-IVUS integrated backscatter intravascular ultrasound, LPA lipid plaque area, PA plaque area. b Correlation between FFR and 3-dimensional classic and IB-IVUS parameters. PV (r = −0.34; p < 0.01) and LPV (r = −0.40; p < 0.01) were significantly correlated with the FFR value. FFR fractional flow reserve, IB-IVUS integrated backscatter intravascular ultrasound, PV plaque volume, LPV lipid plaque volume
Correlation between IVUS parameters and the FFR value. Functional ischemia was defined as ≤0.80. Lesions with functional ischemia had greater PV (186 ± 100 vs. 104 ± 64 mm3), LPV (91 ± 59 vs. 48 ± 45 mm3), and FPV (90 ± 50 vs. 56 ± 41 mm3) compared with lesions without functional ischemia. FFR fractional flow reserve, PV plaque volume, LPV lipid plaque volume, FPV fibrous plaque volume
Representative coronary angiography images and both serial conventional and integrated backscatter intravascular ultrasound images for patients with functional significance (a) and without functional significance (b). White arrow indicates the analyzed segment. The color-coded maps demonstrate lipid (blue), fibrous (green and yellow), and calcified (red) areas. The PV, LPV, FPV, lumen volume, and FFR were 186.6, 119.1, 66.2, 148.2, and 0.68 mm3, respectively, in (a), and 144.9, 43.9, 96.6, 139.7, and 0.81 mm3, respectively, in (b). PV plaque volume, LPV lipid plaque volume, FPV fibrous plaque volume, FFR fractional flow reserve
Discussion
In the present study the major findings were that the LPV and %LPV, as measured by 3-dimensional IB-IVUS, were significantly correlated with the FFR value, and proved to be significant predictors for the FFR value, independent of the various IVUS parameters derived from the classic gray-scale IVUS.
Several previous studies have evaluated the relationship between IVUS parameters and the FFR value. However, the majority of these studies documented the relationship between the FFR value and the QCA and IVUS parameters based on 2-dimensional analysis, such as stenosis length and minimum luminal area [18, 19]. Recently, Yang et al. [20] assessed the relationship between volumetric IVUS parameters and the FFR value and found the positive correlation between plaque volume and the FFR value. In the present study, the lesion length measured by IVUS, minimum luminal area, and PV were significantly correlated with the FFR value in the simple regression model, consistent with the findings of a previous report. Meanwhile, these studies did not take into account the potential impact of plaque morphology.
Theoretically, a pressure drop across the stenosis is related to viscous friction, separation, and turbulence [21]. Therefore, qualitative lesion factors such as plaque morphology as well as quantitative factors could affect the FFR value through producing greater flow resistance and energy loss of the fluid because of viscous friction, separation, and turbulence. Indeed, in a Japanese large-scale prospective multicenter registry study, it was reported that patients with unstable angina pectoris with the frequent development of vulnerable plaques often showed the discrepancy between the FFR value and the QCA as compared to those with SAP [22]. In addition, Versteeg et al. [23] demonstrated that the monocyte toll-like receptors 2 and 4, which are related to plaque vulnerability, were significantly associated with a lower FFR value. These findings suggest the potential impact of plaque characteristics on the FFR value. Nevertheless, in the Fractional Flow Reserve and Intravascular Ultrasound Relationship Study (FIRST), the plaque morphology as measured by virtual histology (VH)-IVUS did not correlate with FFR values [24]. In FIRST, various VH-IVUS parameters were measured only in the 2-dimensional analyses. In the present study, the LPV as measured by the IB-IVUS in the 3-dimensional analysis proved to be an independent predictor for the FFR value. Therefore, the volumetric plaque assessment by IB-IVUS could provide better information in terms of the relationship between plaque morphology and the FFR values as compared to the 2-dimensional analyses. The present findings need further confirmation for any clinical implication of IB-IVUS regarding the feasibility of doing IB-IVUS in a clinical setting.
Study limitations
A limitation of the present study is the recruitment of patients. The patients in this study represented a relatively high-risk population who were scheduled for elective PCI; this may limit the generalization of our results. Arbitrarily excluding lesions with diameter stenosis <25 % might exclude a fair number of truly physiologically significant lesions, thus, recruitment of lesions with diameter stenosis <50 % was an ethical dilemma. In this regard, we evaluated the isolated lesions that have intermediate to severe stenosis (26–90 % of the vessel diameter) in this study. We did not consider the other mild stenosis within the same coronary artery. Therefore, the plaque component at non-culprit lesions might have affected the FFR value. However, we carefully performed a pressure pullback tracing curve to exclude tandem lesions with FFR value stepwise increases of >0.10. Finally, although subjects were prospectively recruited, the number was relatively small. Larger study is needed in this regard.
Conclusions
The lipid volume in lesions with intermediate to obstructive stenosis proved to be an independent predictor for the FFR value. This finding might suggest the contribution of the volumetric plaque morphology contributes to functional ischemia.
References
Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS (2003) Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 107:2900–2907
Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van’t Veer M, Bar F, Hoorntje J, Koolen J, Wijns W, de Bruyne B (2007) Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the defer study. J Am Coll Cardiol 49:2105–2111
Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O’Rourke RA, Dada M, Spertus JA, Chaitman BR, Friedman J, Slomka P, Heller GV, Germano G, Gosselin G, Berger P, Kostuk WJ, Schwartz RG, Knudtson M, Veledar E, Bates ER, McCallister B, Teo KK, Boden WE (2008) Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the clinical outcomes utilizing revascularization and aggressive drug evaluation (courage) trial nuclear substudy. Circulation 117:1283–1291
De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Mobius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engstrom T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Juni P, Fearon WF (2012) Fractional flow reserve-guided pci versus medical therapy in stable coronary disease. N Engl J Med 367:991–1001
Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL (1993) Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation 87:1354–1367
Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJJ, Koolen JJ (1996) Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 334:1703–1708
Tobis JM, Mallery JA, Gessert J, Griffith J, Mahon D, Bessen M, Moriuchi M, McLeay L, McRae M, Henry WL (1989) Intravascular ultrasound cross-sectional arterial imaging before and after balloon angioplasty in vitro. Circulation 80:873–882
Takagi A, Tsurumi Y, Ishii Y, Suzuki K, Kawana M, Kasanuki H (1999) Clinical potential of intravascular ultrasound for physiological assessment of coronary stenosis: relationship between quantitative ultrasound tomography and pressure-derived fractional flow reserve. Circulation 100:250–255
Amano T, Matsubara T, Uetani T, Nanki M, Marui N, Kato M, Arai K, Yokoi K, Ando H, Ishii H, Izawa H, Murohara T (2007) Impact of metabolic syndrome on tissue characteristics of angiographically mild to moderate coronary lesions integrated backscatter intravascular ultrasound study. J Am Coll Cardiol 49:1149–1156
Kawasaki M, Sano K, Okubo M, Yokoyama H, Ito Y, Murata I, Tsuchiya K, Minatoguchi S, Zhou X, Fujita H, Fujiwara H (2005) Volumetric quantitative analysis of tissue characteristics of coronary plaques after statin therapy using three-dimensional integrated backscatter intravascular ultrasound. J Am Coll Cardiol 45:1946–1953
Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF, FAME Study Investigators (2009) Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 360:213–224
Kawasaki M, Takatsu H, Noda T, Sano K, Ito Y, Hayakawa K, Tsuchiya K, Arai M, Nishigaki K, Takemura G, Minatoguchi S, Fujiwara T, Fujiwara H (2002) In vivo quantitative tissue characterization of human coronary arterial plaques by use of integrated backscatter intravascular ultrasound and comparison with angioscopic findings. Circulation 105:2487–2492
Kawasaki M, Hattori A, Ishihara Y et al (2010) Tissue characterization of coronary plaques and assessment of thickness of fibrous cap using integrated backscatter intravascular ultrasound: comparison with histology and optical coherence tomography. Circ J 74:2641–2648
Okubo M, Kawasaki M, Ishihara Y et al (2008) Tissue characterization of coronary plaques: comparison of integrated backscatter intravascular ultrasound with virtual histology intravascular ultrasound. Circ J 72:1631–1639
Okubo M, Kawasaki M, Ishihara Y et al (2008) Development of integrated backscatter intravascular ultrasound for tissue characterization of coronary plaques. Ultrasound Med Biol 34:655–663
Akaike H (1974) A new look at statistical model identification. IEEE Trans Autom Control 19:716–723
Akaike H (1985) Prediction and entropy. In: Atkinson AC, Fienberg SE (eds) A celebration of statistics. Springer, Berlin, pp 1–24
López-Palop R, Carrillo P, Cordero A et al (2013) Effect of lesion length on functional significance of intermediate long coronary lesions. Catheter Cardiovasc Interv 81:E186–E194
Ben-Dor I, Torguson R, Deksissa T, Bui AB, Xue Z, Satler LF, Pichard AD, Waksman R (2012) Intravascular ultrasound lumen area parameters for assessment of physiological ischemia by fractional flow reserve in intermediate coronary artery stenosis. Cardiovasc Revasc Med 13:177–182
Yang HM, Tahk SJ, Lim HS, Yoon MH, Choi SY, Choi BJ, Jin XJ, Hwang GS, Park JS, Shin JH (2014) Relationship between intravascular ultrasound parameters and fractional flow reserve in intermediate coronary artery stenosis of left anterior descending artery: intravascular ultrasound volumetric analysis. Catheter Cardiovasc Interv 83:386–394
Pijls NH (2013) Fractional flow reserve to guide coronary revascularization. Circ J 77:561–569
Nakamura M, Yamagishi M, Ueno T, Hara K, Ishiwata S, Itoh T, Hamanaka I, Wakatsuki T, Sugano T, Kawai K, Akasaka T, Tanaka N, Kimura T (2014) Prevalence of visual-functional mismatch regarding coronary artery stenosis in the CVIT-DEFER registry. Cardiovasc Interv Ther 29:300–308
Versteeg D, Hoefer IE, Schoneveld AH et al (2008) Monocyte toll-like receptor 2 and 4 responses and expression following percutaneous coronary intervention: association with lesion stenosis and fractional flow reserve. Heart 94:770–776
Waksman R, Legutko J, Singh J, Orlando Q, Marso S, Schloss T, Tugaoen J, DeVries J, Palmer N, Haude M, Swymelar S, Torguson R (2013) FIRST: fractional flow reserve and intravascular ultrasound relationship study. J Am Coll Cardiol 61:917–923
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakurai, S., Takashima, H., Waseda, K. et al. Influence of plaque characteristics on fractional flow reserve for coronary lesions with intermediate to obstructive stenosis: insights from integrated-backscatter intravascular ultrasound analysis. Int J Cardiovasc Imaging 31, 1295–1301 (2015). https://doi.org/10.1007/s10554-015-0699-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-015-0699-6