Abstract
Little is known about the impact of duration of ischemia on left atrial (LA) volumes and function during acute phase of myocardial infarction. We investigated the relationship of ischemic times, echocardiographic indices of diastolic function and LA volumes in patients with ST-segment elevation myocardial infarction (STEMI) treated with primary percutaneous coronary intervention (PCI). A total of 433 consecutive STEMI patients underwent echocardiographic examination within 48 h of primary PCI, including the measurement of LA volumes and the ratio of mitral peak velocity of early filling to early diastolic mitral annular velocity (E/e′). Time intervals from onset of chest pain to hospital admission and reperfusion were collected and magnitude of Troponin I release was used to assess infarct size. Patients with LA volume index (LAVI) ≥28 ml/m2 had longer total ischemic time (410 ± 347 vs. 303 ± 314 min, p = 0.007) and higher E/e′ ratio (15 ± 5 vs. 10 ± 3, p < 0.001) than those with LAVI <28 ml/m2, while the indices of LA function were similar between the study groups (p > 0.05, for all). Significant correlation was found between E/e′ and LA volumes at all stages of LA filling and contraction (r = 0.363–0.434; p < 0.001, for all) while total ischemic time along with E/e′ and restrictive filling pattern remained independent predictor of LA enlargement. Increased LA volume is associated with longer ischemic times and may be a sensitive marker of increased left ventricular filling pressures in STEMI patients treated with primary PCI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Left atrium (LA) contributes significantly to total cardiac output with its reservoir, conduit and pumping function. Contribution of LA function to stroke volume is particularly important when left ventricular (LV) diastolic function is impaired and LA dilation has been suggested as a marker of the severity and duration of diastolic dysfunction [1, 2]. Diastolic dysfunction in the setting of acute myocardial infarction (AMI) arises from profound regional asynchrony between ischemic and normal myocardium resulting in disturbed ventricular relaxation and elevated myocardial stiffness [3]. Several studies have demonstrated that LA undergoes significant remodelling following AMI due to increased left ventricular end-diastolic pressure (LVEDP) [4–6] and it has also been shown that LA volume is an important predictor of morbidity and mortality after AMI [7, 8]. On the other hand, the relationship of duration of ischemia and LA volumes during acute phase of myocardial infarction has not been previously investigated.
We therefore investigated the impact of ischemic times on echocardiographic indices of diastolic function and LA volumes in patients with acute ST-segment elevation myocardial infarction (STEMI) treated with primary percutaneous coronary intervention (PCI).
Methods
We retrospectively studied 639 consecutive patients presenting with first-ever STEMI treated with primary PCI in an academic, high-volume centre. Patients were excluded from the analysis if they suffered previous MI or underwent coronary revascularization, received thrombolytics, presented in cardiogenic shock, had significant valvular disease, were in atrial fibrillation or had previously implanted pacemaker. Furthermore, patients in whom primary PCI did not succeed in restoring coronary flow in the culprit artery, also were not eligible for the study. Data regarding time intervals were collected from the patient, the emergency medical service (EMS) team members (report from the patients’ relatives, exact timing of telephone calls to EMS and arrival to hospital) and the cath lab protocol sheets. Any disagreement in reported time intervals was settled by using times reported by EMS and cath lab report sheet. Troponin I assays (peak Troponin I) were collected serially at admission and after 12, 24, 48 h to assess enzymatic infarct size.
Comprehensive transthoracic echocardiogram was performed within 48 h after primary PCI, using Vivid 7 scanner (General Electric, Horton, Norway) and a 2.5- to 5-MHz phased-array transducer. The echocardiographic examination was performed according to American Society of Echocardiography recommendations [9] and included 2-dimensional, color flow, continuous and pulse-wave (PW) Doppler, and tissue Doppler imaging. Detailed measurements were performed offline using dedicated software EchoPac version 11.0 (General Electric, Horton, Norway). Three measurements were made for each parameter and averaged. LV ejection fraction as measurement of systolic function was obtained using the Simpson biplane method of discs. Mitral inflow Doppler and tissue Doppler data were obtained according to guidelines [10]. The ratio of mitral peak velocity of early filling to early diastolic mitral annular velocity (E/e′) was calculated by using the average of the septal and the lateral e′ wave velocity. Diastolic function was graded 0–4 according to guidelines [10]. Left atrial volume was obtained using the Simpson biplane method of discs from apical 4- and 2-chamber views at three time points during heart cycle: (1) maximum volume (LAmax) at the end of systole, right before mitral valve opening; (2) minimal volume (LAmin) at the end of diastole, right before mitral valve closure; (3) volume before atrial contraction (LApreA) acquired at the point of P wave on surface ECG. All volumes are indexed to body surface area (BSA). LA enlargement was defined as LAmax ≥28 ml/m2 (normal ± 1SD) [11, 12]. Mechanical function of LA was calculated based on following formulas: total ejection fraction, LAEF = (LAmax − LAmin/LAmax) × 100; active ejection fraction, as an index of active contraction LAEFa = [(LApreA − LAmin)/LApreA × 100]; passive ejection fraction, as an index of conduit function LAEFp = [(LAmax − LApreA/LAmax) × 100]; LA expansion index, expressing LA reservoire function [(LAmax − LAmin/LAmin) × 100] [13]. Echocardiograms were analysed by an investigator blinded to clinical and angiographic data.
The reproducibility of the measured echocardiographic parameters, (ESV, EDV, EF, E wave velocity, average e′ wave, maximum, minimum and before A wave LA volume index) was tested by two experienced observers and twice by each observer in 20 randomly selected patients. Interobserver coefficients of variation for measuring ESV, EDV, E wave velocity, average e′ wave, maximum, minimum and before A wave LA volume index were 3, 5, 8, 9, 9, 5 and 3 %, respectively. Intraobserver coefficients of variation for repeated same measurements were 3, 5, 6, 5, 7, 4 and 3 %, respectively.
All patients underwent coronary angiography and primary PCI on Siemens Axiom Artis XFA (Siemens, Erlangen, Germany) angiography scanner. Patients were pretreated with loading dose of aspirin (300 mg) and clopidogrel (600 mg), while heparin (80–100 IU/kg), was given before insertion of coronary guidewire. Glycoprotein IIb/IIIa inhibitors were given periprocedurally according to indication by the operator. After PCI aspirin, 100 mg per day, was given indefinitely with clopidogrel, 75 mg per day. Recommended duration of clopidogrel treatment was 12 months. Vascular access, PCI technique, use of guiding catheters, coronary gudiewires, manual thrombus aspiration, predilation and stent implantation were used according to operators’ decision.
A culprit artery was defined as an artery with an identifiable thrombus and/or significant lesion on angiogram corresponding to ECG changes. Coronary artery stenosis was defined as narrowing of the lumen of more than 70 %. Coronary artery blood flow was graded 0–3 according to according to Thrombolysis In Myocardial Infarction (TIMI) scale [14].
Statistical analysis
Continuous variables are presented as mean values ± standard deviation (SD). Categorical variables are presented as percentages. Depending on the distribution of the data, t test or Mann–Whitney test were used to compare continuous variables, whereas Chi square and Fisher’s test were used for categorical variables. Correlation between continuous variables was tested using Pearson’s correlation method. Univariate regression analysis was performed to identify variables associated with LAVI ≥28 ml/m2: the value of p < 0.2 was considered significant and those variables entered Cox multivariate analysis model to determine independent predictors of LA enlargement. p value of <0.05 was considered significant. Statistical analysis was performed using commercially available software (PASW Statistics, version 18, SPSS, Inc., Chicago, IL, USA).
Results
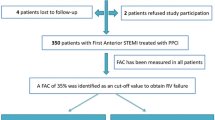
From 639 patients evaluated for inclusion in the study, after accounting for the exclusion criteria, 433 patients were analysed. Patient flow through the study is shown in Fig. 1. Patients were grouped based on maximal LA volume indexed for BSA in the group with enlarged LA (LAVI ≥28 ml/m2) and the group with normal LA volume index (LAVI <28 ml/m2). Clinical characteristics of the study groups are given in Table 1.
Patients from both groups had similar door to balloon time, while those with larger LAVI had longer time intervals from the onset of pain to hospital admission and longer total ischemic time (Table 2).
Angiographic and procedural characteristics were similar in the study groups. There was no significant difference in infarct-related coronary artery, initial TIMI flow, thrombus aspiration and glycoprotein IIb/IIIa inhibitors use and success of the intervention. The study groups had similar maximum values of troponin I representing surrogate of infarct size (Table 3).
The groups had similar LV wall thickness, but demonstrated significant differences in LVEF. In contrast to significant difference in E/e′ ratio between the study groups, indices of LA function (LAEF, LAEF active, LAEF passive, LA expansion index) were similar between the study groups (Table 4).
Significant correlations were found between E/e′ and LA volume and between minimal LA volume and LA volume before atrial contraction (Table 5; Fig. 2).
Patients with severe disturbance of diastolic function (restrictive filling pattern) had higher maximum values of Troponin I representing surrogate of infarct size (55.1 ± 33.7 vs. 32.4 ± 28.7 ng/ml; p < 0.001) (Fig. 3).
In a logistic regression model, including known predictors of LA dilation (smoking, left ventricular ejection fraction, hypertension, thrombus aspiration, maximum troponin I value, average E/e′, grade III diastolic dysfunction and total ischemic time), only total ischemic time, average E/e′ and grade III diastolic dysfunction (restrictive filling pattern) remained independent predictors of LAVI ≥28 ml/m2 (Table 6).
Discussion
Our study demonstrated that STEMI patients with larger LA volumes had longer total ischemic time and higher E/e′ ratio than those with normal LA volumes, while the indices of LA function and infarct size were not related to LA enlargement. On the other hand, restrictive filling pattern (RFP) was not related to ischemic times but was associated with larger myocardial infarctions.
Time from the onset of chest pain to reperfusion is considered as an important prognostic parameter in patients with AMI [15]. Time delays from symptom onset to reperfusion in our study are long, but they are comparable with data from large European registries of acute coronary syndrome [16, 17], as are in-hospital delays from admission to reperfusion [18]. Several studies have shown that Doppler-derived LA filling indices, such as LA volume, E/e′ ratio and restrictive filling pattern, are independent predictors of adverse outcomes in patients with AMI [8, 19–24].
Our findings of LA enlargement with maintained LA function and increased E/e′ ratio suggests that LA volume may be an indicator of an acute rise in LV end-diastolic pressure (LVEDP) in the setting of AMI, besides being well known indicator of chronic elevation of LVEDP. It has been previously reported that LA during AMI responds to volume increase provoked by LVEDP rise by increasing work of contraction, thus maintaining forward LA emptying [25–27]. This mechanism is functional up to certain LA volumes when increased volume load cannot be further compensated by increased work and LA further dilates, indicating that Frank-Starling mechanism is also operative in the LA [28]. Our findings are also in line with study by Bozkurt et al. [4] where echocardiography was performed on admission and after a week, 1 and 3 months, showing that LA volume increased significantly starting from hospital admission. The compensatory mechanism of stroke volume increase responded to volume change, but the difference became significant after a period of 1 month [4]. Left atrial remodeling, defined as increase in LA volume of 8 ml/m2 has been shown to occur in about one fifth of patients after AMI leading to LA functional deterioration over a 12-month period [5].
Previous studies demonstrated that LA volume >34 ml/m2 (normal value ± 2SD) [11, 12] was an independent predictor of adverse events after MI [7, 8]. However, the large LA may not able to further dilate faced with increased LVEDP in MI [25–28] which is why we postulated that mild increase in LAVI (normal ± 1SD or 28 ml/m2) can more accurately reflect initial rise in LVEDP and duration of ischemia.
Our data also suggest that initial rise of LVEDP may cause LA dilatation before restrictive filling pattern develops, indicating that LA volume may be a more sensitive indicator of duration of ischemia than restrictive filling pattern. The potential of LA volume to reflect the increase in LVEDP during AMI has been investigated in the study including more than 600 patients, who underwent simultaneous cardiac catheterization with LVEDP measurement and transthoracic echocardiography [29]. This study demonstrated that maximal and minimal LA volumes and LAEF were associated linearly with LV filling pressures. It was further shown that LA distensibility and LA ejection fraction had logarithmic association with filling pressures and were more accurate in predicting LVEDP >15 mmHg than E/e′ ratio [29].
Nonetheless, it has been recently shown that RFP may also be related to duration of myocardial ischemia [23], which was not confirmed in our study. This discrepancy may be explained by a relatively loose definition of RFP used by Prasad et al. [23] as opposed to strict, guideline-proposed definition used in our study [9, 10]. However, our data confirmed that RFP was associated with larger infarct size in terms of magnitude of troponin I release. Further studies are warranted to determine temporal changes of diastolic function during AMI and to clarify exact relationship between LA volumes and its functional indices and ischemic times in STEMI patients.
Study limitations
Patients were retrospectively evaluated and did not have previous echocardiographic exams which could help to identify those with diastolic dysfunction prior to coronary event. Further, direct measurements of LVEDP by catheterization of the left ventricle were not performed. The effect of standard pharmacological therapy known to influence patients’ outcomes (beta-blockers, ACE inhibitors) was not accounted for, although the guideline-proposed treatment for STEMI, if not contraindicated or poorly tolerated, was given to all patients. Finally, it may be argued that maximum troponin value used in our study may have lower accuracy compared to serial measurements and total troponin release (area under curve) in determining exact infarct size in patients who underwent mechanical reperfusion; however, single-point values, except the ones at admission, have shown good correlation with infarct size estimated by cardiac magnetic resonance imaging or single photon emission tomography [30, 31].
Conclusions
In this retrospective analysis of consecutive STEMI patients treated with primary PCI, greater left atrial volume indexes were associated with longer ischemic times, while functional performance of LA was not impaired. The restrictive filling pattern was associated with larger infarctions, but it was not associated with longer ischemic times.
Abbreviations
- ADM:
-
Hospital admission
- BMI:
-
Body mass index
- BSA:
-
Body surface area
- CABG:
-
Coronary artery bypass grafting
- CAD:
-
Coronary artery disease
- CI:
-
Confidence interval
- CVA:
-
Cerebrovascular accident
- Cx:
-
Circumflex
- EF:
-
Ejection fraction
- LA:
-
Left atrium
- LAD:
-
Left anterior descending
- LAEF:
-
Left atrial ejection fraction
- LAVI:
-
Left atrial volume index
- LV:
-
Left ventricle
- LVEDP:
-
Left ventricular end diastolic pressure
- MI:
-
Myocardial infarction
- OR:
-
Odds ratio
- PAD:
-
Peripheral arterial disease
- PCI:
-
Percutaneous coronary intervention
- RCA:
-
Right coronary artery
- RFP:
-
Restrictive filling pattern
- STEMI:
-
ST-segment elevation myocardial infarction
- SV:
-
Stroke volume
- TIMI:
-
Thrombolysis in myocardial infarction
References
Stefanadis C, Dernellis J, Toutouzas P (2001) A clinic appraisal of left atrial function. Eur Heart J 22:22–36
Hoit BD, Gabel M (2000) Influence of left ventricular dysfunction on the role of atrial contraction. J Am Coll Cardiol 36:1713–1719
Popovic AD, Neskovic AN, Marinkovic J, Lee JC, Tan M, Thomas JD (1996) Serial assessment of left ventricular chamber stiffness after acute myocardial infarction. Am J Cardiol 77:361–364
Bozkurt E, Arslan S, Acikel M, Erol MK, Gurlertop Y, Yilmaz M, Koca H, Atesal S (2007) Left atrial remodeling in acute anterior myocardial infarction. Echocardiography 24:243–251
Antoni ML, ten Brinke EA, Marsan NA, Atary JZ, Holman ER, van der Wall EE, Schalij MJ, Bax JJ, Delgado V (2011) Comprehensive assessment of changes in left atrial volumes and function after ST-segment elevation acute myocardial infarction: role of 2-dimensional speckle tracking strain imaging. J Am Soc Echocardiogr 24:1126–1133
Lamblin N, Fertin M, de Groote P, Bauters C (2012) Incidence, determinants and consequences of left atrial remodelling after a first anterior myocardial Infarction. Arch Cardiovasc Dis 105:18–23
Beinart R, Boyko V, Schwammenthal E, Kuperstein R, Sagie A, Hod H, Matetzky S, Behar S, Eldar M, Feinberg M (2004) Long-term prognostic significance of left atrial volume in acute myocardial infarction. J Am Coll Cardiol 44:327–334
Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, Park SW, Bailey KR, Pellikka PA (2003) Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation 107:2207–2212
Lang RM, Bierig M, Devereux RB, Flachkamp FA, Foster E, Pelikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ (2005) Chamber quantification writing group; American Society of Echocardiography’s guidelines and standards committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from The American Society of echocardiography’s Guidelines and Standards Committee and the Chamber quantification Writing Group, developed in conjunction with European Association of Echocardiography, a branch of European Society of Cardiology. J Am Soc Echocardiogr 18:1440–1463
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22:107–133
Stefano GT, Zhao H, Schluchter M, Hoit BD (2012) Assessment of echocardiographic left atrial size: accuracy of M-mode and two-dimensional methods and prediction of diastolic dysfunction. Echocardiography 29:379–384
Hudsmith LE, Cheng AS, Tyler DJ, Shirodaria C, Lee J, Petersen SE, Francis JM, Clarke K, Robson MD, Neubauer S (2007) Assessment of left atrial volumes at 1.5 Tesla and 3 Tesla using FLASH and SSFP cine imaging. J Cardiovasc Magn Reson 9(673–9):00
Leung DY, Boyd A, Ng AA et al (2008) Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am Heart J 156:1056–1064
TIMI Study Group (1985) The Thrombolysis In Myocardial Infarction (TIMI) trial, phase 1 findings: TIMI Study Group. N Engl J Med 312:932–936
Cannon CP, Gibson CM, Lambrew CT, Shoultz DA, Levy D, French WJ, Gore JM, Weaver WD, Rogers WJ, Tiefenbrunn AJ (2000) Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA 283:2941–2947
Steg PG, Cambou JP, Goldstein P, Durand E, Sauval P, Kadri Z, Blanchard D, Lablanche JM, Guéret P, Cottin Y, Juliard JM, Hanania G, Vaur L, Danchin N, USIC 2000 Investigators (2006) Bypassing the emergency room reduces delays and mortality in ST elevation myocardial infarction: the USIC 2000 registry. Heart 92(10):1378–1383
Di Chiara A, Chiarella F, Savonitto S, Lucci D, Bolognese L, De Servi S, Greco C, Boccanelli A, Zonzin P, Coccolini S, Maggioni AP, BLIT Investigators (2003) Epidemiology of acute myocardial infarction in the Italian CCU network: the BLITZ study. Eur Heart J 24(18):1616–1629
Bradley EH, Herrin J, Wang Y, Barton BA, Webster TR, Mattera JA, Roumanis SA, Curtis JP, Nallamothu BK, Magid DJ, McNamara RL, Parkosewich J, Loeb JM, Krumholz HM (2006). Strategies for reducing the door-to-balloon time in acute myocardial infarction. N Engl J Med 355(22):2308–2320
Nijiland F, Kamp O, Karreman AJ, van Eenige MJ, Visser CA (1997) Prognostic implications of restrictive left ventricular filling in acute myocardial infarction: a serial doppler echocardiographic study. J Am Coll Cardiol 30:1618–1624
Moller JE, Pellikka P, Kober L, Poulsen SH, Nayad O, Torp-Pedersen C (2003) Prognostic importance of systolic and diastolic function after acute myocardial infarction. Am Heart J 145:147–153
Hillis GS, Moller JE, Pellikka PA, Gersh BJ, Wright RS, Ommen SR, Reeder GS, Oh JK (2004) Noninvasive estimation of left ventricular filling pressure by E/e′ is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol 43:360–367
MeRGE-AMI Collaborators (2008) Independent prognostic importance of a restrictive left ventricular filling pattern after myocardial infarction: an individual patient meta-analysis: Meta-Analysis Research Group in Echocardiography acute myocardial infarction. Circulation 117:2591–2598
Prasad SB, See V, Brown P, McKay T, Narayan A, Kovoor P, Thomas L (2011) Impact of duration of ischemia on left ventricular diastolic properties following reperfusion for acute myocardial infarction. Am J Cardiol 108:348–354
Otasevic P, Neskovic AN, Popovic Z, Vlahovic A, Bojic D, Bojic M, Popovic AD (2001) Short early filling deceleration time on day 1 after acute myocardial infarction is associated with short and long term left ventricular remodelling. Heart 85(5):527–532
Braunwald E, Frahm CJ (1961) Studies on Starling’s law of the heart. IV. Observations on the hemodynamic functions of the left atrium in man. Circulation 24:633
Murray JA, Kennedy JW, Figley MM (1968) Quantitative angiocardiography. II. The normal left atrial volume in man. Circulation 37:800
Matsuda Y, Toma Y, Ogawa H, Matsuzaki M, Katayama K, Fujii T, Yoshino F, Moritani K, Kumada T, Kusukawa R (1983) Importance of left atrial function in patients with myocardial infarction. Circulation 67:566–571
Anwar AM, Geleijnse ML, Soliman OII, Nemes A, ten Cate FJ (2007) Left atrial Frank-Starling law assessed by real-time, three dimensional echocardiographic left atrial volume changes. Heart 93:1393–1397
Hsiao SH, Lin KL, Chiou KR (2012) Comparison of left atrial volume parameters in detecting left ventricular diastolic dysfunction versus tissue Doppler recordings. Am J Cardiol 109(5):748–755
Giannitsis E, Steen H, Kurz K, Ivandic B, Simon AC, Futterer S, Schild C, Isfort P, Jaffe AS, Katus HA (2008) Cardiac magnetic resonance imaging study for quantification of infarct size comparing directly serial versus single time-point measurements of cardiac troponin T. J Am Coll Cardiol 51(3):307–314
Kurz K, Schild C, Isfort P, Katus HA, Giannitsis E (2009) Serial and single time-point measurements of cardiac troponin T for prediction of clinical outcomes in patients with acute ST-segment elevation myocardial infarction. Clin Res Cardiol 98(2):94–100
Acknowledgments
Drs. Putnikovic and Neskovic are partly supported by Grant No. 175099 of the Ministry of Science, Republic of Serbia. Unrestricted software support was provided by GE Healthcare.
Conflict of interest
Other authors have no conflict of interest to declare regarding this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ilic, I., Stankovic, I., Vidakovic, R. et al. Relationship of ischemic times and left atrial volume and function in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Int J Cardiovasc Imaging 31, 709–716 (2015). https://doi.org/10.1007/s10554-015-0603-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-015-0603-4