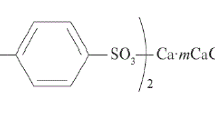
A study was carried out on benzyl alkoxycarbonyl methyl disulfides as multifunctional additives to lubricating oils. Results are given for testing the anticorrosion, anti-wear, anti-microbial action of previously synthesized benzyl alkoxycarbonyl methyl disulfides containing several functional groups. Efficiency was demonstrated for a series of benzyl alkoxycarbonyl methyl disulfides. Depending on their composition and structure, these disulfides improve the anticorrosion, anti-wear, and antimicrobial properties of oils. The antimicrobial properties of these compounds were studied as components of M-11 oil. The synthesized compounds in concentration 0.5-1.5 mass % enhance the resistance of mineral oil to biological damage and also display antimicrobial and antifungal activity. These compounds are more efficient than sodium pentachlorophenolate, which is a commonly used biocide. Testing in a four-ball friction machine indicates that these compounds possess anti-wear properties. Our derivatograph data on the thermal stability of these additives are in accord with the results of thermoanalytical testing of commercial additives.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
There is a steadily growing need for highly-efficient lubricants as well as for improvement of their characteristics in use and methods for obtaining base oils by introducing additives, in particular, by developing a broad assortment of anti-wear and anti-seize additives. Anti-wear and anti-seize additives can reduce the wear on friction surfaces at moderate loads and overcome or moderate seizing under heavy load conditions and high temperature. Organic sulfur compounds are commonly used as such additives [1,2,3,4].
Additives recommended to improve some oil properties often have a negative effect on other properties [5, 6]. Hence, we must find efficient multifunctional additives, which improve a whole set of operational characteristics [7, 8]. Such additives can be compounds that contain several different functional groups in the molecule simultaneously [9,10,11].
An enormous number of organic compounds have been synthesized and extensively tested as such additives. Some organic sulfur compounds have been found to improve the functional properties of oil and can be used as oil additives for this reason [5].
Syntheses have been reported for benzyl alkoxycarbonyl methyl disulfides, whose structure and properties have been studied. These disulfides are known to have anti-wear, anti-seize, and antimicrobial properties [7, 9] and also to enhance the resistance of oils toward oxidation at high temperature. These compounds hold scientific interest since, when introduced as additives, they can form oils with unique functional characteristics [7]. These findings led us to study the antioxidant and anti-corrosion properties of oils with such additives, which generally have a beneficial effect for oils.
Studies of the physicochemical and functional properties of these additives were carried out by standard laboratory methods [12]. The anti-corrosion properties were evaluated on a DK-3 instrument at 140°C for 20 h [13]. The resistance to oxidation was studied on a DK-NAMI instrument at 200°C for 30 h. The anti-wear properties were determined relative to Russian State Standard GOST 9490 on a four-ball CHSHM friction machine using ShKh-15 stainless steel balls with 12.7 mm diameter upon rotation of the shaft at 1420 rpm and relative rate of surface sliding friction 0.54 m/sec [14]. The testing was carried out for 1 h with constant axial load 196 N and the results were evaluated relative to the diameter of the resultant wear spot. In studying the antimicrobial properties of the synthesized compounds, we used M-11 oil, which has already been tested for resistance to the action of mold fungi and bacteria [15,16,17]. The antioxidant properties of the additives were studied by chemiluminescence at 200°C on an instrument featuring an SNK-7 chemiluminescence unit [18, 19]. The heat resistance of the additives was studied on an OD-102T derivatograph with heating in the air at a rate of 5 °C/min. Roasted aluminum oxide served as the standard [20].
Our previous study [14] of the anti-wear and anti-seize properties of these compounds showed that they have good anti-wear properties, which provides for enhancement of the overall anti-wear index and decrease in the wear spot diameter (Figure 1). Disulfides with iso-groups have better anti-wear properties (0.4 mm wear spot diameter) than disulfides with normal groups (0.41 and 0.43 mm). Benzyl alkoxycarbonyl methyl disulfide has greater anti-wear efficiency than dibenzyl disulfide (0.72 mm).
Forbes and Reid [21, 22] proposed a mechanism for this action, in which the RSSR’ molecule initially is adsorbed on the metal surface and then undergoes bond breakage with formation of mercaptides and then mercaptans RSMe and R’SMe.
The formation of a film of ferrous mercaptide proceeds more readily and breakage of two bonds is required for the formation of Fe(SR)2:
These processes account for the anti-wear properties of these compounds because more extensive decomposition, in particular, breakage of the S–S bonds in the disulfides with increasing temperature in the friction zone proceeds to give a modified layer containing sulfur on its surface.
The introduction of an additional sulfide sulfur into the disulfide molecule does not lead to a significant change in the processes occurring on the metal surface and, thus, does not affect the anti-wear properties of disulfides. Due to its free p-electrons, sulfide sulfur can form a ν-molecular complex with metals. Bonds can also form between the sulfide sulfur and metals due to the free metal d-orbitals. These weak bonds cannot significantly affect the anti-wear properties of disulfides.
The disulfides studied presumably are adsorbed on the metal surface initially through the ether group and then the S–S group. The carbonyl group (–OC(O)–) in the disulfide molecules improves their adsorption or chemisorption properties due to the carbonyl group oxygen atom. High efficiency of the compounds studied was demonstrated as anti-wear and anti-seize additives [14].
The study of the thermal transformations of organosulfur compounds with different structures holds interest since they have found common use as additives to lubricating oils. Disulfides reduce the resistance of oils to oxidation upon heating much more efficiently than sulfides [20]. A mechanism involving chemical reactions between the additive molecules and metals proceeding at high friction contact temperatures is directly related to the thermal transformations of the additives leading to a change in the surface properties and, thereby, less wear [23].
The thermal transformations of asymmetrical disulfides have already been studied by thermogravimetry. The thermal transformations of disulfides and their interaction with metals were studied on an OD-102T Paulik-Paulik-Erdey derivatograph system (manufactured in Hungary) [24, 25]. The sample charge for the crystalline products was 100 and 50 mg.
The data for the differential thermal analysis (DTA) with thermogravimetry (TG, DTG) showed that the disulfides have high thermal stability. Figure 2 shows derivatograms for pure benzyl pentoxycarbonyl methyl disulfide.
The temperature, at which the mass loss of the benzyl pentoxy- and benzyl butoxycarbonyl methyl disulfides reaches 50%, is observed on the DTA curve as endo effects at 203 and 218°C for both samples.
The thermographic studies of disulfides permit us, to some extent, to explain the reasons for the differences in the efficiencies of the compounds studied and served as the scientific basis for selecting the more efficient multifunctional additives.
The properties and chemical composition of oil change upon storage and transport, leading to formation of a slime and precipitates due to the metabolic activity of microorganisms, which can produce corrosion of equipment parts [6, 27]. Thus, the quality of oils gradually deteriorates and they become unsuitable for use [28]. Various mechanical and physicochemical methods are employed to combat microbiological corrosion [29]. However, these methods are far from always feasible and, furthermore, none of them provide the efficient protection of oils as the introduction of antimicrobial additives.
In light of the high anti-wear and anti-seize efficiency of the compounds studied, we tested their properties as antimicrobial additives [15,16,17]. Testing of M-11 oil was carried out according to Russian State Standards GOST 9.052-88 and GOST 9.082-77.
Test cultures of bacteria (Mycobacterium lacticola and Pseudomonas aeruginosa), fungi (Aspergillus niger and Cladosporium resinae), and yeast (Candida tropicalis) were used. Meat extract peptone agar was used as the nutritive medium for bacteria, while wort agar was used for the fungi and yeast. The experimental results are given in Figure 3.
Antimicrobial efficiency of benzyl alkoxycarbonyl methyl disulfides at concentrations 0.5% (solid lines) and 1% (dashed lines): I) C6H5CH2SSCH2CO(O)C2H5, II) C6H5CH2SSCH2SSCH2CO(O)C3H7, III) C6H5CH2SSCH2CO(O)C3H7-i, IV) C6H5CH2SSCH2CO(O) C4H9, V) C6H5CH2SSCH2CO(O)C4H9-i, VI) C6H5CH2SSCH2CO(O)C5H11, VII) sodium pentachlorophenolate, 1) bacteria, 2) yeast, 3) fungi
The compounds studied at concentrations 0.5-1.5 mass % efficiently suppress the growth of microorganisms. Their antimicrobial activity (1.4-2.6 cm-1) is higher than that of the industrial additive sodium pentachlorophenolate (1.3-1.6 cm-1), which was used as a standard The other compounds gave results similar to the standard. Negative changes in the functional properties of oils are not observed at concentration 0.5-1.5%, while the antiseptic action of these compounds is retained at elevated humidity and temperature for a prolonged period. The compounds studied do not have a deleterious effect on the physicochemical properties of oils.
Oils from low-sulfur petroleum with a high content of paraffin hydrocarbons forming aggressive organic acids upon oxidation, which react with nonferrous metals and their alloys, have a greater tendency to promote extensive corrosion. Anticorrosion additives protect anti-friction materials such as leaded bronze, forming a strong protective film on their surface [30]. Anti-corrosion additives such as zinc dithiophosphates used in most motor oils do not protect alloys of silver and phosphor bronze against corrosion and actually actively facilitate their corrosion at high temperature. Special oils not containing zinc dithiophosphates must be used in motors, in which such anti-friction materials are used. With this in mind, we investigated the disulfide compounds studied here as anti-corrosion additives.
In order to elucidate the dependence of the anti-corrosion properties of these additives on their composition and structure, they were tested in M-11 mineral oil. The corrosion activity of the oils with these additives was studied on a DK-3 instrument according to Russian State Standard GOST 11063-64 [13]. The mass loss of a copper (g/m2) plate served as the determining criterion:
where m is the mass loss from the plate over the testing time, g.
Thus, the corrosion activity upon the introduction of our additives (1.5 mass %) to M-11 oil was reduced from 180 to 58-1.54 g/m3. The testing results are given in Figure 4.
Our testing showed that the greatest anti-corrosion activity among the compounds studied is found for benzyl butoxy- (from 124 to 27 g/m2) and benzyl iso-butoxycarbonyl methyl disulfides (to 1.54 g/m2), which virtually eliminates the corrosiveness of M-11 oil. On the other hand, benzyl ethoxy- (to 56 g/m2), benzyl pentoxy- (to 36 g/m2), and benzyl isopropoxycarbonyl methyl disulfides (to 13.2 g/m2) hardly affect the anti-corrosion properties of the oil, which apparently is related to the instability of these compounds in the presence of oxygen at high temperature. The anti-corrosion activity of the oil is reduced to 24.6 g/m2 upon the addition of the same amount of the additive, DF-11.
The most important property of lubricating oils for extending the operational lifetimes of motors is their resistance to oxidation at high temperatures. The change in oil quality mainly occurs due to the action of atmospheric oxygen and high temperatures of the metal surfaces. There have been reports in the literature about antimicrobial efficiency of some organic sulfur compounds. In order to confirm this behavior, a series of the compounds studied was tested in lubrication oils as antioxidant additives. The antioxidant properties of these compounds were studied using chemiluminescence at 200°C on a USK-7 instrument [18, 19]. This method is based on weakening of the oil luminescence by the antioxidant due to the action of peroxide radicals.
A quantitative relationship exists between the antioxidant activity of oxidation inhibitors and their capacity to suppress chemiluminescence emission [19]. Oxidation inhibitors react with peroxide radicals, reducing their concentration. Since the recombination of peroxide radicals is specifically responsible for chemiluminescence emission, a decrease in the concentration of peroxide radicals will decrease the luminescence intensity [31,32,33]. The relative antioxidant efficiency of an inhibitor is calculated using the following formula
wwhere Eae is the relative antioxidant efficiency and J/J0 is the ratio of the chemiluminescence emission intensities over elapsed time during the experiment with inhibited and non-inhibited oxidation of the oils.
We tested an industrial antioxidant, namely, butylated hydroxytoluene, for comparison as a standard under analogous conditions. The relative antioxidant efficiency of the compounds studied is given in Figure 5, which shows that these compounds as 0.5 mass % and 1 mass % additives to Vaseline oil display antioxidant activity. The chemiluminescence emission is 70-80%, i.e., is on the same level as the industrial additive, butylated hydroxytoluene. Increasing the concentration to 1 mass % leads to greater antioxidant efficiency with enhanced chemiluminescence emission to 82%.
Antioxidant properties of the compounds studied at concentrations: 0.5% (dashed line) and 1% (solid line) in Vaseline oil: I) C6H5CH2SSCH2CO(O)C2H5, II) C6H5CH2SSCH2CO(O)C3H7, III) C6H5CH2SSCH2CO(O)C3H7-i, IV) C6H5CH2SSCH2CO(O) C4H9, V) C6H5CH2SSCH2CO(O)C4H9-i, VI) C6H5CH2SSCH2CO(O)C5H11, VII) butylated hydroxytoluene
The greatest antioxidant properties among the compounds studied are found for benzyl pentoxycarbonyl methyl disulfide. Almost no precipitate formation or greater viscosity is noted in the presence of this compound.
Therefore, the compounds studied have considerable useful properties and can be seen as multifunctional additives to mineral oils. The introduction of these additives to M-11 oil in 1.5 mass % concentration considerably improves its anti-corrosion, antioxidant, antimicrobial and anti-wear properties. A definite correlation is found between the efficiency of these properties and their molecular structure. The multifunctionality and high efficiency of the operational properties of these additives discovered in this work may be attributed to the presence of disulfide and carbonyl groups, long alkyl chains, and their intramolecular synergism.
References
T. Kh. Akchurina, B. I. Musaeva, I. P. Ismailov, et al., Azerbaidzhanskii Khimicheskii Zhurnal, No. 4, 108-112 (2014).
T. Kh. Akchurina, D. Sh. Gamidova, B. I. Musaeva, et al.,, Al’ternativnye Istochniki Syr’ya. Sbornik Nauchnikh Trudov, No. 4, 10-18 (2020).
S. A. Kuznetsov, S. G. Ivanova, and N. I. Koltsov, Vestnik Chubashskogo Universiteta, No. 2, 48-51 (2009).
V. Kontham, K. V. Padmaja, and D. Madhu, Journal of Saudi Chemical Society, 942-954 (2020).
A. M. Kuliev, The Chemistry of Oil and Fuel Additives [in Russian], Khimiya, Leningrad (1985).
Sh. R. Aliev, Khimiya i Tekhnologiya Topliv i Masel, No. 3, 36-39 (2012).
G. Yu. Kolchina, O. Yu. Poletaeva, É. M. Movsumzade, et al., Bashkirskii Khimicheskii Zhurnal, 27, No. 1, 27-31 (2020).
I. G. Anisimov, K. M. Badyshtova, S. A. Bnatov, et al., Fuels, Lubricant Materials, Industrial Liquids. Assortment and Application [in Russian], Tekhinform, Moscow (1999).
A. N. Agaev, S. M. Velievaaaa, N. N. Zeinalova, et al., Khimiya i Tekhnologiya Topliv i Masel, No. 3, 33-35 (2012).
S. M. Velieva, I. D. Kulaliev, É. M. Dzhavadova, et al., Azerbaidzhanskii Khimicheskii Zhurnal, No. 4, 578-583 (2018).
V. D. Sukhoverkhov and I. M. Vasil’kevich, Mir Nefteproduktov, 6, 31-34 (2008).
Oil Products: Oils. Lubricants. Additives, Part 3, Standard Publ., Moscow (1987), pp. 144-147.
Russian State Standard GOST 11063-64. Motor Oils with Additives. Methods for Determining Stability According to NAMI Petroleum Products Research Institute [in Russian].
USSR Inventor’s Certificate 1,512,119.
F. Y. Aliev and S. M. Azizova, Processes of Petrochemistry and Oil-Refining, 15, No. 1, 15-20 (2014).
Russian State Standard GOST 9.082-77. Oils and Lubricants. Methods of Laboratory Testing for Resistance to the Action of Bacteria [in Russian].
Russian State Standard GOST 9.052-88. Oils and Lubricants. Methods of Laboratory Testing for Resistance to the Action of Mold [in Russian].
R, F. Vasil’ev, O. K. Karpukhn, and V. Ya. Shlyapintokh, Zhurnal Fizicheskoi Khimii, 35, 461-467 (1961).
N. M. Emanuél’ and Yu. N. Lyaskovskaya, Suppression of the Oxidation of Fats [in Russian], Pishchepromizdat, Moscow (1961).
F. Yu. Aliev and R. A. Mamedova, Science and Applications Conference. Abstracts [in Russian], Elm, Baku (1988), p. 3.
Yu. S. Zaslavskii and R. N. Zaslavskii, Mechanism for the Action of Anti-Wear Additives to Oils [in Russian], Khimiya, Moscow (1978).
E. S. Forbes and A. I. Reid, ASLE Transactions, 16, No. 1, 50-60 (1973).
Z. Z. Khairullina, Method of Thermal Analysis. Instructions for Laboratory Work [in Russian], Kazan National Research Technological Institute, Kazan (2020).
F. Paulik. I. Paulik, and L. Erdey, The Derivatography. Theoretical Fundamentals [Russian translation], Hungarian Optical Factory, Budapest (1974).
V. I. Almyashev, Thermal Methods of Analysis [in Russian], St. Petersburg Electrotechnical University, St. Petersburg, Russia (1999).
S. N. Litvinenko, Protection of Petroleum Products Against the Action of Microorganisms [in Russian], Khimiya, Moscow (1974).
V, M. Farzaliev, M. T. Abbasova, Z. K. Soltanova, et al., Azerbaidzhanskii Khimicheskii Zhurnal, No. 2, 26-29 (2006).
L. M. Khurnova, Obrazovanie i Nauka v Covremennom Mire. Innovatsii, No. 2, 280-292 (2019).
Ya. M. Paushkin, I. A. Rabotnova, G. P. Vishnyakova, et al., Prikladnaya Biokhimiya i Mikrobiologiya, No. 6, 778-731 (1970).
A. V. Belyakov and V. M. Kremeshnyi, Lubricating Compositions with Enhanced Anti-Corrosion and Anti-Wear Properties [in Russian], All-Russian Thermotechnical Research Institute, Russian Federation Application 2002104540/04 (2003).
USSR Inventor’s Certificate 741118.
V. Ya. Shlyapintokh, O. N. Karpukhin, L. M. Postnikov, et al., Chemiluscence Methods for Studying Slow Chemical Processes [in Russian], Nauka, Moscow (1966).
I. F. Rusina, O. N. Karpukhin, and O. T. Kasaikina, Khimicheskaya Fizika, 32, No. 8, 49-64 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 3, pp. 43–48, May – June, 2024.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aliev, F.Y., Azizova, S.M. Efficiency of the Action of Multifunctional Additives in Lubricating Oils. Chem Technol Fuels Oils 60, 535–540 (2024). https://doi.org/10.1007/s10553-024-01709-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-024-01709-7