Technology of the synthesis of carbon materials from the most common carbohydrates in nature, glucose and cellulose, is discussed. The chemical transformations of the carbohydrates during carbonization were investigated using thermogravimetric analysis and FTIR-spectroscopy. The influence of the precursor and the pretreatment process on the structure and morphology of carbon materials was shown. The morphology of materials obtained from glucose and cellulose pretreated in air is characterized by large irregular particles. In turn, the pretreatment of carbohydrates under hydrothermal conditions makes it possible to obtain materials consisting of microspheres.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Carbon materials are widely used in power engineering as a heat source and energy storage sources [1]. Currently, an urgent task is to develop a technology for the production of carbon materials from renewable natural resources. Various carbohydrates can be considered as such sources [2]. As such, for example, cellulose or biomass of plant origin has a number of advantages, such as low cost and ubiquity [3, 4].
The technology for producing various carbon materials is based on pyrolysis in an inert atmosphere at elevated temperatures. Various carbon-rich sources can act as precursors for pyrolysis. The pyrolysis stage is often preceded by a pre-treatment of the precursor. One of the widespread pretreatment methods is the carbonization of precursors in air [5]. However, a number of authors note that the pre-treatment of the precursor under hydrothermal conditions contributes to the production of carbon materials with a completely new morphology – spheres or plates with different sizes [6]. It should be noted that carbon materials obtained during carbonization in air or under hydrothermal conditions are widely used for products such as biochar [6], as well as electrode materials for supercapacitors [7] and metal-ion batteries [8].
In this work, we investigated the properties of carbon materials obtained from D-glucose (RUSHIM) and cellulose (Avicel® PH101, Sigma Aldrich) after their pretreatment in air or under hydrothermal conditions. After pretreatment, the solid precursor was annealed at 1300 °C in a tubular furnace in an argon flow. The resulting precursors and carbon materials were investigated using IR spectroscopy. To designate the materials, the following names were adopted – UMG-1 and UMG-2 for materials obtained from glucose after pretreatment in air or under hydrothermal conditions, respectively, and UMC-1 and UMC-2 for cellulose materials. The structure and morphology of carbon materials were investigated using powder X-ray diffraction and scanning electron microscopy. The changes occurring during the annealing of glucose, cellulose, and precursors obtained from them were investigated using thermogravimetry (TGA) and differential thermal analysis (DTA).
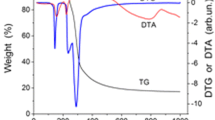
Annealing in air was used as the first type of conversion for glucose and cellulose. According to the data of [9], upon annealing in air, carbohydrates lose their crystal structure and in the course of various reactions (dehydration, polycondensation, etc.) transform into an amorphous carbon-containing product. Fig. 1a shows the results of TGA/DTA of the glucose pretreatment process. The data in Fig. 1a indicate that below 170°C mass loss of glucose was 5%, which can be attributed to the process of surface dehydration. With further heating of glucose, melting occurs, followed by deeper dehydration and the formation of a glassy product. The resulting product was used to synthesize UMG-1 carbon material. When cellulose is heated in air, two peaks are observed corresponding to exothermic processes accompanied by mass loss (Fig. 1b). The greatest mass loss was observed at temperatures above 300°C. For the synthesis of UMC-1 carbon material, a cellulose precursor obtained at 320°C was chosen.
Hydrothermal carbonization has been considered as an alternative pretreatment process. To carry out the process, an aqueous solution of glucose or an aqueous suspension of cellulose were placed in an autoclave and subjected to carbonization at 200°C. Materials UMG-2 and UMC-2 were obtained from a solid washed product after hydrothermal carbonization of glucose and cellulose using pyrolysis. Fig. 2 shows the results of TGA/DTA of pyrolysis of precursors from glucose and cellulose obtained after hydrothermal carbonization. During pyrolysis at 1300 °C in an inert atmosphere, carbon precursors of this type lose about 50% of their weight.
To study the chemical transformations of materials during pyrolysis, the obtained precursors and carbon materials were investigated by IR spectroscopy (Fig. 3). It can be noted that high-temperature annealing at 1300 °C in an inert atmosphere leads to the production of a material with a lower concentration of functional groups.
In general, in IR spectra, carbon materials exhibit a lower intensity of functional groups in comparison with the studied precursors. The absorption bands at 3420 and 1750 cm–1 correspond to vibrations of carboxyl and carbonyl groups [10]. The presence of such bands can be associated with the partially oxidized surface of carbon materials. It should be noted that the 3420 cm–1 band was more pronounced for glucose carbon materials. The 2350 cm–1 band is associated with the presence of CO2 in the air.
For the IR spectra of precursors obtained from cellulose, the presence of a vibrational band in the region of 1630 cm–1, corresponding to the vibrations of the C=C bond, was noted, which may indicate the presence of aromatic rings in the structure. This phenomenon can confirm the partial carbonization of cellulose during pretreatment. The intensity of this band was higher for precursors derived from glucose, which may indicate deeper carbonization during the pretreatment. The rather low intensity of the halo in the region of 3420 cm–1 is the result of the dehydration process, since glucose and cellulose before heat treatment have a broad and intense peak in the region of 3000–3700 cm–1 [11].
The resulting carbon materials were examined using scanning electron microscopy and showed a significant difference in morphological characteristics (Fig. 4). Carbon material obtained from glucose pretreated in air is large particles with an indefinite shape and macropores with an average diameter of 50 μm. In turn, the morphology of the material obtained in the course of hydrothermal carbonization (Fig. 4b) is presented in the form of aggregates of microspheres with a diameter of less than 10 μm.
Materials obtained from cellulose have a more indefinite morphology, but it is noteworthy that the morphology of materials, in general, does not change dramatically depending on the pretreatment method. Aggregation of fine particles with an average diameter of less than 1 μm (Fig. 4d) may indicate the initial stage of cellulose degradation under hydrothermal conditions. At the same time, it is noted in [10] that a deeper hydrothermal destruction of cellulose occurs at temperatures above 210°C [10]. Thus, deeper carbonization can also lead to the formation of aggregates of microspheres, as is the case with hydrothermal glucose carbonization.
The structure of carbon materials was investigated by powder X-ray diffraction (Fig. 5). Three broad reflections are present in the X-ray diffraction patterns of carbon materials, which is typical of carbon materials with a turbostratic structure. The first halo at 21.7–23.2° characterizes the interplanar spacing d002 in pseudographitic domains. For the UMG-1 and UMG-2 materials, this distance was 3.97 and 3.72 Å, respectively, for the UMC-1 and UMG-2 materials, 4.01 and 4.09 Å, respectively. It can be noted that the materials are characterized by a large interplanar spacing in comparison with ideal graphite, the interplanar spacing of which is 3.35 Å.
Thus, the investigation shows that the pretreatment process significantly affects the morphology of carbon materials. Hydrothermal carbonization makes it possible to obtain spherical materials with an average diameter of up to 10 μm. At the same time, materials from pretreated cellulose showed similar characteristics regardless of the pretreatment method, which may indicate insufficient destruction of the hydrocarbon during carbonization. All studied materials are characterized by a high degree of disorder and a low content of functional groups. The data can be useful for further targeted design of carbon materials for various applications.
The study was financed by the Russian Foundation for Basic Research as part of the scientific project No. 19-33-90112.
References
Deborah Duen Ling Chung, Carbon Materials: Science and Applications, Vol. 3, World Scientific (2019).
He Mingyuan, Yuhan Sun, and Buxing Han, Angew. Chem. Int. Edit., 52, Is. 37, 9620–9633 (2013).
M. S. Kotelev et al., Chem. Tech. Fuels Oils, 53, No. 5, 722–726 (2017).
A. A. Novikov, B. M. Anikushin, D. A. Petrova, et al., Chem. Tech. Fuels Oils, 54, 564–568 (2018).
I. C. Lewis, Carbon, 20, No. 6, 519–529 (1982).
M. Antonietti and М. М. Titirici, CR Chim., 13, Is. 1–2, 167–173 (2010).
M. Inagaki, F. Kang, M. Toyoda, et al., MRS Bulletin, 39, 1018 (2014).
K. Maksymiuk, J. Stroka, and Z. Galus, Chemistry, Electrochemistry, and Electrochemical Applications| Lead, 762–771 (2009).
Jiang Bin et al., J. Agr. Food Chem., 56, Is. 13, 5138–5147 (2008).
V. Tucureanu, A. Matei, and A. M. Avram, Crit. Rev. Anal. Chem., 46, Is. 6, 502–520 (2016).
M. Sevilla and A. B. Fuertes, Chem – Eur. J., 15, Is. 16, 4195–4203 (2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 6, pp. 3–6, November – December, 2021.
Rights and permissions
About this article
Cite this article
Bobyleva, Z.V., Apostolova, M.O., Lakienko, G.P. et al. Features of the Synthesis of Functional Carbon Materials from Plant Carbohydrates. Chem Technol Fuels Oils 57, 871–875 (2022). https://doi.org/10.1007/s10553-022-01320-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-022-01320-8