The deformation—strength (strength and plasticity) and dilatometric properties (contraction or volume shrinkage) of binary composites of food paraffin P-1 and the polymers low-pressure polyethylene, polyethylene waxes, atactic polypropylene, and copolymers of ethylene and vinylacetate are investigated. The structural and mechanical properties of the polymer—paraffin composites are plotted as functions of composition. The dependences of these properties on the content of modifying component in the P-1 composites are compared.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Polymers play an important role in contemporary life by supplying modern technology with new, inexpensive, and promising materials [1,2,3,4,5]. Composites of oil paraffin with polymers, ceresins, waxes, and other modifiers of its disperse structure are used in various economic sectors (food, packaging, agriculture, radioelectronics, engineering, etc.) [6,7,8,9,10]. Thus, polymer-paraffin composites are used to store and ripen cheeses in the cheese-making industry and to cultivate grafted plants in viticulture and horticulture [6, 7]. Also, they should form a thin, strong, and rapidly solidifying water-impermeable film on the surface of the preserved product. The paraffin composites should have the required set of operating properties, which are determined by their composition and crystalline disperse structure, depending on the application area, each of which imposes certain quality requirements on them.
Structural and mechanical properties of paraffinic oil products were reported before by us [11]. That research found that high-molecular-mass polymers affected the structural and mechanical properties of food paraffin. The ability to control the formation of the polymer—paraffin disperse structures because of the need to create commercial polymer—paraffin composites with given properties has now become critical. This work was especially significant for the systematized review of the structural and mechanical properties of polymer—paraffin composites that was published by us for the first time and provided a scientific basis for designing new materials with given structural and mechanical properties.
Food paraffin grade P-1 from Ozeksuatskoe oil was used as the base to prepare composites with the polymer components. Paraffin P-1 and the polymers were melted together at temperatures 20°C greater than the dissolution temperature of the polymers in the paraffin. Paraffin P-1 had melting point 7; TS= 328.3 K and solid-state hexagonal-rhombic If H→R phase transition temperature TH→R= 309.1 K. By comparison, synthetic ri-C24H50 had TS = 323.7 K and TH→R= 319.2 K.
Thermal properties were determined using dilatometry, differential thermal analysis (DTA), and thermal x-ray diffraction. Structural and mechanical properties (strength Pm, volume shrinkage or contraction AV, plasticity 𝜀m/Pm) were measured on a specially designed laboratory stand, the construction specifics and study method for which were discussed by us before [II]. Strength was determined at 293 K\( \left({\mathit{\Pr}}_m^{293}\right) \); volume shrinkage (contraction), in the temperature range from the start of crystallization to 293 K\( \left(\Delta {V}_{T_s}^{293}\right) \); plasticity, at 293 K. Penetration (depth of needle penetration into solid paraffin) was determined as an additional physicomechanical characteristic of the composites. This standard method for measuring hardness within the limits of GOST 25771-83 was selected by us because of its wide acceptance.
Polymers used to formulate binary mixtures with paraffin P-1 included low-pressure polyethylene (LPPE), polyethylene waxes, a tactic polypropylene (APP) [12], and copolymers of ethylene and vinylacetate (EVA) [13]. LPPE of base grade 273 from Budenovskii Chemical Combine had melting point 427 K and molar mass 4,250 g/mol. Polyethylene waxes (PW) were prepared in two ways, i.e.. via thermal destruction (PW-25, PW-100, PW-300, PW-800, PW-1000) and during polyethylene manufacturing at various plants. The molecular mass of the thermal destruction PWs varied from 850 to 4,000 g/mol. PW E-114 (Germany) and samples from Grozny State Petroleum Technical University and Gur'ev Chemical Plant (GCP) had molecular masses of 1,010, 1,500, and 2,600 g/mol, respectively. Their dropping points were 369-378 K. EVAs (TU N 6-05-1636-97) differed in vinylacetate (VA) content (11, 28, 40-44 wt.%). APP (TU 6-05-194-80) with an irregular steric structure had dropping point 433 K (GOST 6793-74).
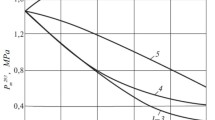
As a rule, polymers are stronger than solid oil paraffins. Adding them to paraffin helps to increase the strength of the polymer—paraffin composite disperse structure. Figures 1 and 2 compare the strength properties of solid polymer—paraffin composites with the studied polymers (LPPE, PW-300, APP, and EVA with 11 and 28% VA) at concentrations up to 10 wt.%. Adding all these polymers except APP helped to increase the strength of the polymer—paraffin composites.
Only APP weakened the paraffin disperse structure. It weakened significantly the disperse structure of its composite with paraffin P-1. The strength of the disperse structure with 10% APP in the paraffin composite was 1.07 MPa vs. 1.45 MPa for pure P-1. The strength was weakest with 5% APP. The strength of the polymer—paraffin composites decreased more sharply for APP concentrations up to 5 wt.% and then less significantly.
The other studied polymers were placed in the following order of increasing strengthening action: PW-300, LPPE, EVA (28% VA), EVA (11% VA). LPPE and EVA (28 and 11% VA) up to a concentration of 5 wt.% had practically the same strengthening action on P-1. Concentrations of EVA (11% VA) >5 wt.% strengthened the composites more than EVA (28% VA) and LPPE (Fig. 1).
PWs were less effective at strengthening the disperse structure of the paraffin composites. The strength of their disperse structure began to increase more sharply only if the PW molecular mass increased to >3,500 g/mol, reaching 2.8 MPa for PW-1000 with a molecular mass of 4,000 g/mol (Fig. 3).
Therefore, PW-300 (mol. mass 2,400 g/mol) and PW-1000 (mol. mass 4,000 g/mol) at a concentration of 10% in oil paraffin gave extremely insignificant composite strength increases of 0.1-0.15 MPa (Figs. 1, 3, and 4). Conversely, the composite strength decreased sharply to 3.5 MPa if such weak PWs as PW-300 and PW GCP were added (10%) to very strong synthetic n-C24H50 (\( {P}_m^{293} \) = 5.4 MPa).
EVA (11 wt.% VA) was the most effective strengthener of the paraffins according to Figs. 1, 5, and 6. EVA (28% VA) increased the strength of the composites with oil paraffin and synthetic n-C24H5O slightly less than EVA (11% VA).
Thus, the presence of VA groups in EVA was observed to affect its strengthening action on the paraffin disperse structure. P-1 composites with EVAs containing from 2.5 to 40 wt.% VA groups were studied to define this effect (Fig. 5).
The results showed that the composite paraffin disperse structure was strengthened most to 1.9 MPa for EVA with 12 wt.% VA groups (Fig. 5, solid line). The strengthening action decreased smoothly and significantly if the VA content in the EVA was increased above 15 wt.% because of the decreasing strength of the EVA itself (the tensile strength of EVA decreased from 15 to 5 MPa if the VA content was increased from 5 to 30%). Thus, the paraffin composite with 10 wt.% EVA (40% VA) was only 0.15 MPa stronger than the paraffin itself. The strength increased from 1.5 to 3.8 MPa if the EVA (28% VA) concentration in the paraffin composite was increased from 1 to 50 wt.% (Fig. 6). The strengthening was greatest for EVA concentrations above 30 wt.%.
Hence, the studied modifiers of solid paraffin P-1 disperse structure could be divided into strengthening and weakening groups. The first group included PW-300 and PW GCP (\( {P}_m^{293} \)= 1.55 MPa), EVA (40% VA) (1.6 MPa), EVA (28% VA) and LPPE (1.7 MPa), and EVA (11% VA) (1.9 MPa). The strengths of the paraffins with 10 wt.% of the modifier are given in parentheses. The second group included PW-25 (1.2 MPa) and APP (1.07 MPa).
As a rule, modifiers that increased the strength of the paraffin composites increased their hardness as evaluated from the penetration depth of a needle and vice versa (Fig. 1 as compared to Fig. 2). The less the penetration was, the greater the hardness was.
The polymeric modifiers were placed in the following order of increasing effect on the hardness of the paraffins: EVA (28% VA) (9.10-4m), PW-300 (8.7.10-4m), EVA (11% VA) (8.2.10-4m), PW GCP (7.5.10-4m), and LPPE (6.5.10-4m). A comparison of the effects of these polymeric modifiers on the strength and hardness of the polymer—paraffin composites showed that highly effective strengthening did not always correspond to increased hardening. For example, composites with PW-300 and PW GCP were weaker than those with EVA (28% VA) although they were harder than them. The same conclusion could be drawn by comparing the strength and hardness of oil paraffins with oil ceresins. Thus, the strength of oil paraffins was 0.8-1.4 MPa with penetrations varying from 32.10 to 13.1e in. respectively. Penetrations ranged from 28 to 16.10m for oil ceresins with \( {P}_m^{293} \) = 0.2-0.6 MPa, i.e., much weaker (by 2-6 times) ceresins had penetrations and: therefore, hardnesses that were practically the same as those of the paraffin.
Thus, penetration may be the only physicomechanical quality parameter of paraffin oil products limited by a GOST but it does not always correlate with the strength. Therefore, it cannot replace the latter for evaluating crystal structure specifics and intermolecular interactions determining their strength. Penetration characterizes to a certain extent the tendency of the crystal structure to undergo plastic deformation.
The penetration should be greater for softer and more plastic crystals and those packed less densely. Polymeric products (PWs and others) consisting of higher molecular mass hydrocarbons than paraffins have finer crystalline disperse structures with more densely packed crystals. Small plate-like wax crystals consisting of many layers should be less susceptible to needle penetration (deformation) than larger plate-like paraffin crystals with fewer layers. This is also the major factor for measuring the hardness of paraffin composites. Paraffin composites with the wax of highest molecular mass PW-1000 (mol. mass 4,000) at concentrations >40 wt.% had the highest hardness at 298 K (penetration <5-10-4 m). Waxes of lower mol. mass (<1,500) or with low contents in the composites (<10%) had less effect on the paraffin structure.
The polymeric products were plasticizers of the paraffin disperse structure (Fig. 7). Plasticizing action was evaluated from the plasticity of the solid polymer—paraffin composite. PW-300 was the most effective plasticizer.
The polymeric modifiers decreased contraction of the paraffin composites and most significantly at concentrations up to 10 wt.% (Fig. 8). This was consistent with the disordering action of the modifiers, regardless of their nature, on the paraffin crystal structure.
Separate crystallization first of high-melting and then low-melting components in the R- or H-systems typical of them at this temperature was characteristic of paraffin composites with polymeric products and oil wax-ceresin modifiers. Solid oil paraffin P-1 crystallized first in the H- and then in the R-system. Polymers adopted only the R-system. Binary composites of solid oil paraffin with polymeric products at 293 K were heterogeneous systems consisting of two rhombic-system phases. Combining P-1 with polymeric products helped to reduce and if the added polymers had parameters less than those of the paraffin and vice versa.
References
E. B. Sviridov and V. K. Dubavyi, Book of Polymers: Properties and Applications, History and Status of Materials Based on High-Molecular-Mass Compounds [in Russian], Izd. Politekhnicheskogo Univ., St. Petersburg, 2015, 546 pp.
U. Reifenhauser, Tara Upakovka, No. 1, 4449 (2010).
H. G Karian (ed.), Handbook of Polypropylene and Polypropylene Composites, Marcel Dekker Inc., New York, 2003, 740 pp.
M. Biron, Thermoplastics and Thermoplastic Composites: Technical Information for Plastic Users, Elsevier Science, 2007, pp. 240-259.
A. N. Pereverzev and E. N. Kipriyanova, Paraffin Composites. Review Information. Series: Oil Refining [in Russian]. TsNIITEneftekhim, Moscow, 1990, No. 8.
Protective Coatings for Cheeses. Meat and Cheese Industry: Review Information [in Russian], TsNIITEImyasomolprom, 1971.
E. A. Aleksandrova, R M. Gergaulova, G A. Sbramko, et al., RU Pat. 2,349,071, Jan. 1, 2009, "Method of fall wheat treatment."
Z. A. Kochan, Use of Microcrystalline Waxes for Paper and Cardboard Treatment [in Russian], Khimiya, Moscow, 1967,31 pp.
N. F. Bogdanov and L.A. Parfenova, Khim. Tekhnol. Topl. Masel, No. 1,7-10 (1976).
I. G Anisimov, Neftepererab. Neftekhim., No. 3, 21-23 (1981).
A. S. Abubakarova, Zh. T. Rhadisova, E. A. Aleksandrova. et al., "Study of structural-mechanical properties of paraffm-containing petroleum products."Khim. Tekhnol. Topl Masel, No. 2, 38-41 (2014).
V. P. Nekhoroshev, E. A. Popov, A. V. Nekhorosheva, et al., Plast. Massy. No. 12, 6-9 (2005).
E. V. Veselovskaya, N. I. Severova, and F. I. Duntov, Ethylene Copolymers [in Russian], Rhimiya, Leningrad, 1983, 224 pp.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya ropily i Masel, No. 4, pp. 30 — 34, July — August, 2019.
Rights and permissions
About this article
Cite this article
Aleksandrova, E.A., Khadisova, Z.T., Aklimadova, K.K. et al. Structuraland Mechanical Properties of Polymer—Paraffin Composites. Chem Technol Fuels Oils 55, 404–411 (2019). https://doi.org/10.1007/s10553-019-01045-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-019-01045-1