The goal of this work was to determine the effectiveness of various surfactant-based reagents and disclose the interrelationships of the effectiveness of the reagent and the type of the soil being cleaned, the concentration of the working reagent solution, and the alkalinity of the solution. It was found that reagents containing anionic and nonionic surfactants and their working solutions having high pH values are most effective. However, this parameter is not decisive for both petroleum hydrocarbons and natural organic compounds. Study of the influence of sorption capacity of soil matrices on the effectiveness of soil cleaning by reagent treatment revealed that sorption of molecules of contaminating matters and molecules of surfactants markedly reduces the overall effectiveness of leaching out of organic matters from the soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Contamination of environmental objects with oil and oil products is currently one the major issues of ecological safety. Soils degraded by oil lose their economic value, the soil profile changes, and morphological and chemical properties of the soil are transformed. Analysis of zonal soils showed that contaminants alter the whole array of physicochemical properties of soils and that brown, floodplain, and alluvial soils, which are capable in the end of self-cleaning and self-regeneration are more resistant to oil attack [1]. The risk of the soil OCS treatment conditions were also determined: 40-95C (preferably 60°C), volume ratio of the soil to be cleaned and aqueous surfactant solution must not be less than 1:1.
The selection of the type and grade of the surfactant for OCS cleaning must be based on various criteria: effectiveness, ecological safety, and economic feasibility. The goal of this work was to determine the effectiveness of various types of surfactants and to study the degree of influence of various factors on the process.
Several laboratory tests were conducted in accordance with the procedure in [4] to determine the effectiveness of surfactants recommended for soil cleaning. A general assessment was also made of the leaching capacities of the reagents in terms of reduction of the content of natural organic compounds in the soil.
A list of the studied surfactants is given in the table 1.
The effectiveness of OCS cleaning was determined for several model OCS:
-
standard soil prepared in conformity with ISO 11268-2 (oil content 5 and 10 wt. %); • chernozem (black soil) from the Voronezh region (oil content 10 wt. %);
-
Peter Peat grade zhirnozem (rich soil) based on top and bottom peat with addition of agroperlite, river sand, limestone flour (oil content 10 wt. %).
For modeling oil contamination of soils, the following were used:
-
stock-tank oil of the Moscow Oil Refinery (MOR) having a density of 0.92 g/cm3;
-
crude oil from TsPS Varyegan field having a density of 0.84 g/cm3.
Laboratory determination of surfactant effectiveness was carried out by mixing OCS with aqueous solutions of the reagents in conical flasks on a shaker under the following conditions:
working solution temperature at the start of mixing, °C | 65 |
OCS:solution wt. ratio | 1:10 |
mixing rate, rpm | 120 |
treatment time. min | 30 |
After settling the suspension for 24 h, the free oil on the liquid surface was collected mechanically and the used surfactant solution was withdrawn by a pump. The suspension left was washed several times with fresh pipe water and then the sample was dried on a Buchner funnel using a filter paper.
The sample was prepared by the EPA 3545 method employing a Thermo Scientific Dionex ASE 150 extractor and using spectrally clean n-hexane as the solvent. The analysis was carried out by the PND F 16.1:2.21-98 (M 03-03-2012) standard procedure.
Study of the solubilizing capacity of the surfactant with respect to natural organic compounds consisted in determination of the change in the organic matter content following GOST 23740-2016 after extraction of the uncontaminated Zhirnozem peat soil with aqueous surfactant solutions. The extraction was carried out for 15 min in conical flasks on a laboratory shaker and the solutions of the reagents were preheated to the working temperature 60-65°C.
The diagram depicting the effectiveness of reduction of oil products content in model soils containing 10% stock-tank oil from the Moscow Oil Refinery is shown in Fig. 1 for several reagents. The surfactants BOK-6, AddiMax PV01, Vega ChM, and Stenor25R15E10 were found to be most effective. The working solutions of these surfactants had high pH values (3 11).
Note as well that the reagent Stenor 25R15E10 having a neutral medium of the working solution exhibited high effectiveness of standard soil cleaning from oil contamination, but its effectiveness with respect to oil-contaminated chemozem remained at the 36-37% level. This is explained perhaps by the fact that the standard soil consisting 69 wt. % of sand sorbs oil hydrocarbons to a lesser extent and retain them primarily by adhesive and cohesive forces. Nonetheless, this proposition is of a limited nature because the said trend does not persist for several other surfactants, such as OP-10, Neftenol ML, and Sulfonol NP-1.
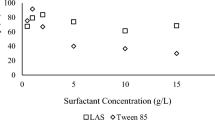
The influence of working solution concentration on the effectiveness of cleaning of model peat soil with 10% MOR oil was determined for the four most effective reagents (Fig. 2).
As shown in Fig. 2, the effectiveness of model soil cleaning increases also with increase of working surfactant solution concentration. At the same time, use of more concentrated working reagent solutions is not always economically beneficial and is also fraught with potential risk of secondary environmental pollution. So, we investigated the effectiveness of working surfactant solutions with concentrations of 1 and 5 wt. %. For the reagent BOK-6, a concentration of 4 wt. % is optimum because increase of its effectiveness at 5 wt. % concentration is negligible. Diminution of oil products content was determined for a standard soil contaminated with the lighter oil of the TsPS Varyegan field in quantities of 5 and 10 wt. %. The experimental results are presented in Fig. 3.
A comparative analysis of Figs. 1 and 3 shows that the overall effectiveness of cleaning of standard soil from lighter oil is higher than from heavier oil. It is evident from Fig. 3 that the trend of increase of OCS cleaning effectiveness persists with increase of working surfactant solution concentration. Also observed is a direct relationship of increase of surfactant cleaning effectiveness with fall of the initial oil contamination level. The only exception is BOK-6 that exhibits equally high effectiveness of cleaning of standard soil with 5 and 10 wt. % contamination.
At the next stage of the investigations we determined the degree of leaching from the soils of natural organic matter that accompanies the oil hydrocarbons solubilization process. This parameter is important for evaluating the effectiveness of reagents based on synthetic surfactants because, first, humic matters play an important part in soil self-cleaning processes, for they bind high-toxicity elements without hindering further natural processes of detoxification of matters [6], and, second, humic matters, humic acids in particular, also possess surface-active properties, which may potentially increase the OCS cleaning effectiveness.
The contamination-free peat-based zhirnozem soil with a potentially high content of humic matters was used as the initial soil and 1 N aqueous NaOH solution, which is often used along with soda ash as a degreasing agent, was used as the comparison substance.
Since the maximum OCS cleaning effectiveness is achieved at alkali values of pH of aqueous solutions of reagents, in all probability, humic acids, which determine the soil fertility and the self-cleaning capacity, are preeminently leached out during reagent treatment of the soils [6, 7].
The results of determination of the degree of leaching of natural organic matters from uncontaminated peat soil due to extraction with aqueous surfactant solutions characterized by various pH values of the medium are presented in Fig. 4.
As can be seen from Fig. 4, solubilization of natural organic matters depends a great deal on the alkalinity of the used reagent solutions. However, the surfactants AddiMax PV01 and PV02 are characterized by solubilizing capacity with respect to humic matters at solution pH of 11.22 and 11.23, respectively. Thus, alkaline medium of working surfactant solutions is one of the determinants, but not the only factor of the capacity of some reagents to leach out humic matters from the OCS.
Investigation of the capacity of some anionic and nonionic surfactants to leach out oil and natural organic compounds indicated significant influence of pH of the working solution on the reagent treatment effectiveness index. Also notable are the differences in solubilization of oil products for different soil matrices (peat, chemozem, sand + clay, etc.), which, in all probability, is attributable to two factors, namely, the sorption capacity of the soil and the natural reagent that affects the capacity of the surfactant molecules to be sorbed by the soils. Thus, according to the published data [6, 7], anionic surfactants are characterized by lesser capacity to be sorbed than nonionic surfactants. This property of the reagents affects the quantity of surfactant molecules capable of interacting with the contaminant and so the overall effectiveness and the required concentration of the working solution for attaining the claimed soil cleaning quality index.
Simultaneously with the fact that a part of the surfactant molecules may be "consumed" for solubilization of natural organic matters, in fertile soils with high contents of humic acids, the later, in the OCS treatment process, especially at high pH values of the working aqueous solution, will also act as surfactants, enhancing thereby the overall OCS cleaning efficiency.
Thus, OCS treatment is characterized by simultaneous occurrence of various physicochemical processes that affect the effectiveness of soil cleaning by surfactant-based reagents. It is pressing and essential to conduct further investigations of various surfactants to determine the important criteria of surfactant selection for undertaking soil reclamation measures that ensure effective soil cleaning from oil contamination with adherence to the principles of ecological safety.
References
A. F. Tumanyan, N. V. Tyutyuma, A. N. Bondarenko, et al., Chem. Technol. Fuels and Oils, 53, No. 3, 369-376 (2017).
A. F. Tumanyan, E. K. Vatovskaya, and N. V. Tyutyuma, Chem. Technol. Fuels and Oils, 49, No. 2, 180-186 (2013).
D. I. Khalilova and D. M. Yunusova, Byul. Rezult. Nauchn. Issled., No. 1-2, 23-31 (2017).
Russian Federation Patent 2244685.
E. A. Beznozdreva, D. S. Vorob'ev, L. G. Emel'yanova, et al., Collection of Innovative Solutions of Biodiversity Conservation for Oil Refining Sector [in Russian]. 000 RA IL'F, Moscow (2015), p. 275.
A. A. Izosimov, Physicochemical Properties, Biological Activity, and Detoxifying Capacity ofMimic Compounds Differing in Origin of Organic Matter [in Russian], Biological Science Candidate’s dissertation, Moscow (2016).
A. I. Popov, Humic Matters: Properties, Constitution, Formation [in Russian], Izd. S., St. Petersburg (2004), p. 248.
NFESC Technical Report TR-2206-ENV Surfactant-Enhanced Aquifer Remediation (SEAR) Design Manual [Electronic resource]/EOS Remediation [official site], URL: http://www.eosremediation.corn/download/Source%20Zones/SEAR/SEAR%20Design%20Manual.pdf (accessed: 09.10.2018).
A. Zubair, Design and optimization of surfactant based enhanced remediation of bunker fuel contaminated soil, St. John's, Newfoundland, Canada (2015), p. 182.
The authors express their gratitude to Dr. V A. Terekhova, Professor, M. V. Lomonosov Moscow State University, for supplying the samples of chernozem of the Voronezh Region, ISO 11268-2 standard soil, and crude oil of the TsPS Varyegan field.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya r Tekhnologiya Topliv i Mani, No. 6, pp. 47-51, November — December, 2018.
Rights and permissions
About this article
Cite this article
Kulikova, O.A., Mazlova, E.A., Bradik, D.I. et al. Use of Surfactant-Based Reagents for Cleaning Oil-Contaminated Soils. Chem Technol Fuels Oils 54, 743–750 (2019). https://doi.org/10.1007/s10553-019-00982-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-019-00982-1





 - chernozem.
- chernozem.

 ) light oil of Varyegan field.
) light oil of Varyegan field.
 -decrease in organic matter content,%.
-decrease in organic matter content,%.