The properties of conventional shale hydration inhibitors were studied using samples of Longmaxi shale. The inhibition was evaluated by the rolling recovery test, linear swelling rate, and clay interlayer spacing. The results indicate that K2SiO3 inhibits clay hydration far better than other inorganic shale inhibitors while polyamine is superior to other organic shale inhibitors of clay hydration. Alkaline solution is more effective in suppressing shale dispersion. A neutral environment has little impact on this parameter. On the contrary, an acidic medium can even promote it. Most of the inhibitors are incapable of suppressing simultaneously clay dispersion and expansion. Therefore, they should be used in conjunction to suppress synergistically clay hydration, dispersion, and expansion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Shale is found in about 75% of the formations drilled worldwide and is responsible for approximately 90% of wellbore instability problems [1]. Drilling problems associated with shale swelling and dispersion include bit balling, hole enlargement, caving, and even abandonment of the well, which naturally have a major impact on the drilling efficiency and subsequent economic outlays [2,3,4,5,6]. Despite these problems, the petroleum industry uses water-based drilling fluids as much as possible because they are relatively cheap and more environmentally friendly than oil-based drilling fluids [7]. Therefore, inhibitors in water-based drilling fluids that are used to suppress shale hydration and maintain wellbore stability are some of the most significant elements of successful drilling operations.
Various chemicals have been used to minimize hydration and swelling of shale. One of the earliest and most common methods is based on the addition of inorganic salts such as KCl, NaCl, and other cationic materials to drilling fluids [8,9,10].
Recently, much effort has been devoted to developing new shale inhibitors, especially organic ones. The number of inhibitors [11,12,13,14,15] used in drilling [16, 17] was greatly expanded by the emergence of polyoxypropylene diamines, polyether diamines, quaternary ammonium salts, polyoxyalkyleneamines, and poly(oxypropylene)—amidoamines. However, there is still not a simple or general fluid for controlling the stability of shale formations [18] because systematic comparisons of the used inhibitors and studies of the relationships among the evaluation methods are rare. This is not conducive to using all capabilities to suppress shale hydration.
The present investigation comprised series of experiments including rock mineral analyses, rolling recovery tests, linear swelling rate measurements, and clay interlayer spacing determinations that were conducted to compare the inhibitory properties of the shale inhibitors and oil-based drilling solutions and to characterize Longmaxi shales. Also, correlations among these methods were studied to understand better the mechanism of action and to offer subsequently theoretical guidance for the application of shale inhibitors.
Longmaxi shale was obtained from the outcrop in southwestern China. Sodium montmorillonite (Na-MMT) was a natural smectic aluminosilicate obtained from Xiazijie Bentonite Co., China. Chlorides of Ca, K, and Fe(III) and CaO were provided by Kelong Chemical Reagent Co., Ltd., China. Potassium sorbate was purchased from Wang Long Group Co., Ltd., China. Mineral oils were provided by Maoming City Baorun Chemical Co., Ltd., China. Potassium silicate (K2SiO3) was purchased from Aladdin Industrial Corp. Polyamine, cationic polymer, emulsified asphalt, and oil-based muds were provided by Oilfield Co. All experimental materials were used without further purification.
The roller oven and linear swelling meter were purchased from Qingdao Tongchun Oil Instrument Co., Ltd., China. X’pert x-ray diffraction equipment was provided by PANalytical Corp., Holland.
Whole rock mineral analysis was performed according to the Chinese standard of analysis method for clay minerals and ordinary non-clay minerals in sedimentary rocks by x-ray diffraction (SY/T 5163-2010).
Rolling recovery was determined by transferring shale samples (~50 g, 6-10 mesh) to a container. After that, inhibitor solutions of different concentrations were added. The samples were placed into a roller oven at 105°C for 16 h. The shale particles were rinsed with H2O, passed through a 40-mesh sieve, and dried to constant mass in an oven at 105°C. Rolling recovery was calculated using the formula:
where M is the amount of shale after the cycle.
Interaction between the aqueous fluid and shale was also evaluated by a Fann LSM (linear swell meter). Shale samples (~5 g) were placed into a hydraulic cylinder compressor and pressurized at 5,000 psi for 5 min. Afterwards, the samples were immersed in distilled H2O, solutions of inorganic and organic inhibitors, and oil-based drilling fluids and left to stand for 24 h. The linear swelling rates (e) were calculated using the formula:
where ΔL is the change of sample length and L, the initial sample length.
Interlayer spacing was measured by x-ray diffraction (XRD) analysis. Bentonite (2 g) was added to inhibitor solution (100 mL), stirred for 30 min, and left to stand for 24 h. Then, the dispersion was centrifuged for 30 min. The precipitate was collected and used for XRD measurements. Interlayer spacing was determined using Bragg’s equation:
where d is the interlayer spacing; θ, scanning angle; and ε, x-ray wavelength.
Figure 1 shows XRD patterns of ordinary Longmaxi shale.
Results from rock mineral analyses of Longmaxi shale are given below:
Content, % |
quartz ............................................................... 23.76 |
feldspar ............................................................. 3.33 |
plagioclase ........................................................ 3.79 |
calcite ............................................................... 19.68 |
Ankerite ............................................................. 4.02 |
pyrite .................................................................. 1.72 |
amine feldspar ....................................................... 0 |
clay minerals ................................................... 43.75 |
kaolin .................................................................. 0 |
chlorite .......................................................... 26.3 |
illite .............................................................. 65.05 |
mixed (I/S) ...................................................... 8.65 |
Interlayer ratio (I/S) ........................................ 10 |
Longmaxi shales have high contents of brittle minerals such as quartz so that they are brittle in general. The fraction of montmorillonite in a mixed-layer with illite was 10%, which illustrated that Longmaxi shales could hydrate, disperse, and swell. These shales were chosen to study the properties of shale inhibitors for just this reason.
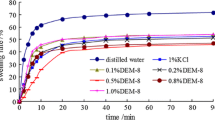
The rolling recovery test simulates not only the high temperatures of downhole conditions but also rolling of drilling fluids. In view of this, the test is frequently used by researchers to study the properties of shale rock inhibitors [13]. Figure 2 shows the test results.
Figure 2a shows that the rolling recovery of the shale samples in distilled H2O was 69.16%, which demonstrated that the Longmaxi shale may have hydrated and dispersed. In general, K2SiO3, KCl, CaO, and KCl + CaCl2 were better inhibitors than distilled H2O. K2SiO3 was the best inhibitor with a rolling recovery of 97.56%. In addition, KCl and CaO also showed excellent inhibiting performance. However, the rolling recovery for CaCl2 was slightly lower than that of distilled H2O; for FeCl3, much less at only 29.91%. Considering that the excellent inhibiting performance of KCl resulted from its low hydration energy and small hydrated ion [20] and that K2SiO3 and CaO hydrolyze to give an alkaline solution whereas FeCl3 gives an acidic one, we concluded that an alkaline environment suppressed shale dispersion more effectively than a neutral solution, which had practically no effect. Conversely, an acidic solution could even promote dispersion.
Figure 2b shows that all organic inhibitors were more effective than distilled H2O. Oil-based drilling fluids gave the best results by practically completely preventing dispersion. Potassium sorbate had the highest rolling recovery and confirmed the positive influence of an alkaline environment. In addition, the rolling recovery was greater for the cationic emulsified asphalt than for the uncharged emulsion, indicating that the cations in the first instance played a role similar to that of potassium ions. The test showed that the rolling recovery of the combined KCl and emulsified asphalt was greater than that of emulsified asphalt alone, suggesting that organic inhibitors combined with KCl were more efficient shale inhibitors. This agreed with previous research results [20].
Linear swelling rate measurements can intuitively reflect hydration and swelling of shale on a macroscopic scale so that they are widely used to evaluate shale inhibitors [21]. Figure 3 shows the test results.
Linear swelling rates of inorganic (a) and organic inhibitors (b): 1) distilled H2O; 2) 1% CaCl2; 3) 5% K2SiO3; 4) 5% FeCl3; 5) 5% KCl; 6) 1% CaO; 7) 5% KCl + 1% CaCl2 (a); 1) distilled H2O; 2) 3% polyamine; 3) 3% cationic polymer; 4) 5% potassium sorbate; 5) 5% emulsified asphalt; 6) mineral oil; 7) oil solution; 8) 5% KCl + 5% emulsified asphalt (b).
Figure 3a shows that the swelling rate of shale in contact with distilled H2O was 13.37%, which indicated that Longmaxi shales exhibited hydration swelling. The plots show that CaCl2 did not reduce hydration and swelling, leading to swelling slightly lower than that in distilled H2O. The degree of inhibition was greater when a shale core was treated with 5% KCl solution than with 1% CaCl2 solution. Conversely, CaO and FeCl3 did not suppress but promoted shale swelling. The reason could be the high calcite content (19.68%). The main component of calcite is calcium carbonate, which can react with hydrogen ions produced by hydrolysis of Fe3+ to shift the equilibrium toward formation of iron hydroxide. It can be seen clearly that K2SiO3 prevented dispersion most effectively with a linear swelling rate of about 3.23%.
Figure 3b shows that shale samples in contact with distilled H2O had the highest percentages of linear swelling after 24 h of testing. It can be seen that potassium sorbate did not reduce significantly hydration and swelling. Significant reductions were also not observed for the 3% ionized polymer and 5% KCl + 5% emulsified asphalt solutions. The degrees of swelling were slightly less than that of distilled H2O. Treatment of a shale core with 5% emulsified asphalt solution, white oil, and oil-based drilling fluids gave values less than 3%, consistent with excellent inhibiting performance. The percentage linear swelling for a shale core in contact with 3% polyamine solution was 7.71%, which was significantly reduced as compared with distilled H2O. Besides, the swelling rate increased little and remained less than that of KCl after immersion in polyamine solution for 24 h, illustrating that polyamine played the role of a clay stabilizing agent.
The interlayer space is enclosed between two adjacent clay layers and can reflect inhibiting properties on a microscopic level. Figure 4 shows XRD patterns of Na-MMT/inhibitor with a wet sample.
X-ray diffraction analysis of Na-MMT/inhibitors with wet samples for inorganic (a) and organic inhibitors (b): 1) distilled H2O; 2) 1% CaCl2; 3) 5% K2SiO3; 4) 5% FeCl3; 5) 5% KCl; 6) 1% CaO; 7) 5% KCl + 1% CaCl2 (a); 1) distilled H2O; 2) 3% polyamine; 3) 3% cationic polymer; 4) 5% potassium sorbate (b).
Interlayer spacings (E) in the clay structures are given below:
Distilled H2O ........................................................................................................... 19.43 |
1% CaCl2 ........................................................................................................... 19.06 |
5% K,SiO3 ........................................................................................................... 15.83 |
5% FeCl3 ........................................................................................................... 19.45 |
5% KCl ........................................................................................................... 14.71 |
1% CaO ........................................................................................................... 15,68 |
5% KCl+1% CaCl2 ................................................................................ 14.72 |
3% Polyamine ........................................................................................................... 14.62 |
3% Cationic polymer ........................................................................................................... 14.31 |
5% Potassium sorbate ........................................................................................................... 15.24 |
Figure 4 shows that the peak moved to the right as compared with that of distilled H2O, indicating that the interlayer spacing decreased. The further to the right it moved, the stronger the inhibiting properties of one agent or another were. The interlayer spacing decreased when the shale sample contacted the inhibitors, indicating that clay hydration could be suppressed by them.
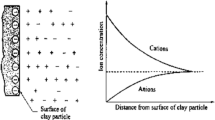
Inorganic inhibitors suppressed clay hydration and swelling mainly by ion exchange and compression of the electrical double layer. The interlayer spacing decreased in the order FeCl3, CaCl2, KCl, which demonstrated that the swelling suppression order was K+ > Ca2+ > Fe3+. The reason is that the diameter of K+ is the same as that of O atoms in rings of hexagonal clusters of Si-–O tetrahedra in montmorillonite. The K+ ion easily entered the hexagonal network and inhibited hydration expansion via fixation of the lattice and reduction of the interlayer spacing [19]. The interlayer spacings were 14.71 E for 5% KCl solution and 15.83 for 5% K2SiO3 solution, which was less than that for distilled H2O. Also, the interlayer spacing decreased significantly for 3% polyamine and 3% cationic polymer solutions, suggesting that they could suppress clay hydration and be used as hydration inhibitors.
According to the experimental results, dispersion, hydration, and swelling could be practically completely suppressed upon contact of a sample with oil-based fluids. However, aqueous fluids are often used in shale formations in view of the high costs and environmental restrictions on oil-based fluids [7]. Considering the rolling recovery test results and the linear swelling rates, K2SiO3 can be proposed as the agent giving the best hydration inhibition among the inorganic alternatives whereas polyamine was best among the organic ones. Therefore, they are recommended for use as inhibitors when working with aqueous drilling fluids in shale formations with rolling recovery >90% and linear swelling rate <10%.
Figure 5 shows plots for analyzing linear correlations between the rolling recovery, linear swelling rate, and interlayer spacing of the clay structures. The Pearson correlation coefficient was used to describe the linear correlation between the parameters and described how closely related the distance between the two given variables was:
where R is the Pearson correlation coefficient; N, number of samples; and X i and Y i , independent quantities. was stronger for greater absolute values (±1) of the linear correlation coefficient and weaker the closer it was to 0.
Figure 5a shows that the rolling recovery decreased with increasing linear swelling rate. The R2 of the line fitting was 0.71, which was indicative of a good linear correlation between the linear swelling rate and the shale rolling recovery. This illustrated that most of the inhibitors could not simultaneously suppress dispersion and swelling. Therefore, they must be used in conjunction to generate synergy. Also, Figure 5b shows that the interlayer spacing decreased with increasing rolling recovery. This was essentially the same correlation as between the linear swelling rate and the rolling recovery because swelling can be considered a macroscopic manifestation of the interlayer spacing, i.e., the linear swelling rate increased as it increased. Figure 5c supported this.
The following conclusions could be drawn from the experimental results.
An alkaline environment was more effective for suppressing shale dispersion. A neutral solution had practically no effect. Conversely, an acidic environment promoted unwanted dispersion.
K2SiO3 exhibited the best hydration inhibition among the inorganic inhibitors whereas polyamine was the best among the organic inhibitors. Most of the inhibitors could not simultaneously suppress dispersion and swelling so that they had to be used in conjunction to generate synergy.
References
R. P. Steiger and P. K. Leung, SPE Drill. Eng., 7, 181-185 (1992).
C. Lu, SPE 14249, SPE Annual Technical Conference and Exhibition, Las Vegas, Nevada, September 22–25 (1987).
J. J. Sheu and A. C. Perricone, SPE 18033, SPE Annual Technical Conference and Exhibition, Houston, Texas, USA, October 2-5 (1988).
R. L. Anderson, H. C. Greenwell, J. L. Suter, et al., Ann. Braz. Acad. Sci., 82, 43-60 (2010).
I. E. O. Onuoha, H. I. Bilgesu, and S. Ameri, SPE 149271, SPE Eastern Regional Meeting, Columbus, Ohio, August 17-19 (2011).
L. A. Richard, H. C. Greenwell, J. L. Suter, et al., Ann. Braz. Acad. Sci., 82, 43-60 (2010).
D. G. Nunes, A. P. M. Da Silva, J. Cajaiba, et al., J. Appl. Polym. Sci., 131 (2014).
R. Caenn and G. V. Chillingar, “Drilling fluids: state of the art,” J. Pet. Sci. Eng., 14, 221-230 (1996).
E. Stamatakis, C. J. Thaemlitz, G. Coffin, et al., “A new generation of shale inhibitors for water-based muds.” SPE 29406, SPE/IADC Drilling Conference, Amsterdam, Netherlands, February 28-March 2 (1995).
E. Van Oort, “On the physical and chemical stability of shales,” J. Pet. Sci. Eng., 38, 213-235 (2003).
A. D. Patel, E. Stamatakis, and E. Davis, US Pat. 6,484,821, Nov. 26, 2002.
R. Schlemmer, A. Patel, J. Friedheim, et al., AADE 2003 National Technology Conference, Houston, America, April 1-3 (2003).
H. Y. Zhong, Z. S. Qiu, W. A. Huang, et al., Energy Sources, Part A, 35, 218-225 (2013).
H. Y. Zhong, Z. S. Qiu, W. A. Huang, and J. Cao, Appl. Clay Sci., 67-68, 36-43 (2012).
Y. Z. Qu, X. Q. Lai, L. F. Zou, and Y. N. Su, Y.N., Appl. Clay Sci., 44, 265-268 (2009).
S. Young and G. Ramses, SPE/IADC 103967, Indian Drilling Technology Conference and Exhibition, Mumbai, India, October 16–18 (2006).
J. Marin, F. Shah, M. Serrano, et al., SPE 120768, SPE Latin American and Caribbean Petroleum Engineering Conference, Cartagena, Colombia, May 31-June 3 (2009).
A. Patel and S. Gomez, SPE 164108, SPE International Symposium on Oilfield Chemistry, Texas, USA, April 8-10 (2013).
R. K. Clark, Water-Soluble Polym., 10, 171-181 (1986).
R. C. Balaban, E. L. F. Vidal, and M. R. Borges, Appl. Clay Sci., 105, 124-130 (2015).
S. Y. Liu, X. G. Mo, and C. G. Zhang C.G., J. Dispersion Sci. Technol., 25, 63-66 (2004).
Acknowledgments
The study was financially supported by the 973 Project (Major State Basic Research Development Program of China, No. 2013CB228003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 6, pp.99 – 104, November – December, 2017.
Rights and permissions
About this article
Cite this article
Cao, C., Pu, X., Wang, G. et al. Comparison of Shale Inhibitors for Hydration, Dispersion, and Swelling Suppression. Chem Technol Fuels Oils 53, 966–975 (2018). https://doi.org/10.1007/s10553-018-0886-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-018-0886-y