Graphical dependences of the temperatures of crystallization, start and end of crystallization, and of phase hexagonal-rhombic H → R conversion in the solid state on wax compositions of petroleum paraffin P-1 with ceresines (C-65, C-80), wax (ZW-1), and soft paraffin (SP) are proposed. It is shown that the melting points of paraffin-ceresine mixtures obey an additivity rule provided that the melting points of the mixed components do not differ by more than 15°C. Paraffin P-1, soft paraffins, and waxes crystallize initially in the H and then also in the R phase, and ceresines only crystallize in the R phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Solid paraffin-containing petroleum products (paraffins, ceresines, waxes) find widespread use in various branches of industry and agriculture. Here they are used both as commercial products under various brands and as paraffin composites (PC) with various additives. Rational utilization of paraffin petroleum products and also the production of paraffin compositions with ceresines as waxes of given quality require targeted investigation of their operational characteristics (melting or boiling points, strength, plasticity, contraction) in relation to their proportions in the mixtures. Here polymorphous modification of their crystalline phase under various temperature conditions is of great importance.
Paraffins are characterized by two crystalline phases: high-temperature hexagonal (H) or α phase (low strength and high plasticity); low-temperature rhombic (R) or β phase (strong and brittle). The established dependence of the structural and mechanical operational properties of paraffin-containing systems on their phase state gives rise to the need for phase analysis of these petroleum products [1, 2]. The principal aim of the present work was thus to determine the relationships governing the effect of the composition of paraffin composites on their melting points T m, setting points T s, and hexagonal–rhombic H → R transformation points T H → R .
Structure formation in paraffin composites with decrease of temperature begins with the appearance of the first crystals of solid phase and ends with the formation of a three-dimensional dispersed structure. This process can be divided into three stages:
-
I.
Formation of the nuclei of crystals, which was defined by the temperature at the beginning of crystallization T c.
-
II.
Accumulation of solid phase with simultaneous structure formation. The maximum temperature at which the three-dimensional dispersed structure is formed corresponds to the setting point (T s). The setting point T s or melting point T m is determined from the loss of fluidity (in a simplified version at the ball of the thermometer).
-
III.
Prolongation of structure formation from T m to the end of crystallization T ec, when all the liquid phase has passed into the solid state.
-
IV.
Transition of the paraffin crystals from the hexagonal H system (𝛼 phase) to rhombic R (T H → R ) or β phase. This transition is more characteristic of pure n-alkanes and solid petroleum paraffins and often does not appear in more complex mixtures.
-
V.
Cooling of the crystalline rhombic R or β phase to a given cooling temperature (T 0).
For the comparison of paraffins, ceresines, and waxes their temperature characteristics were defined by a single characteristic: melting temperature T m, K (GOST 4255-75) or setting temperature T s, K (GOST 2287-74). The temperature limits of the phase transformations T c and T ec and also T H → R for composites of the paraffin petroleum products and their mixtures were determined by thermographic and dilatometric methods on specially created laboratory apparatus that provides programmed cooling of the investigated system and makes it possible to record “temperature–time” and DTA curves automatically. The provision of a slow cooling rate 0.01-0.05 deg/s made it possible to approximate the crystallization conditions to equilibrium. The obtained results were monitored by a thermo x-ray diffraction method; the method developed by S. K. Filatov for phase analysis of polycrystals was used [3]. The reproducibility of the results from measurement of the temperatures of the phase transformations of petroleum paraffins P-1, V2, and T and synthetic n-C24H50 by the dilatometric method, confirmed by differential thermal analysis (DTA), was not less than ±0.2 deg. It should be noted that the temperature at the end of crystallization T ec or beginning of melting T m, determined by thermographic and dilatometric methods, almost coincided with the temperatures at the beginning of the appearance of x-ray amorphism in these systems during heating, established by thermo x-ray diffraction [4, 5]. By thermo x-ray diffraction it was also possible to confirm the reliability of the employed dilatometric and DTA methods during measurement of the temperatures of the R → H transitions in the paraffin products and their mixtures.
Taking account of the considerable differences in the temperature characteristics of the paraffins and ceresines [1, 5, 6], we investigated the regularity and features of the variation of these characteristics during compounding of the paraffin petroleum products with each other. During preparation of the paraffin compositions as base we used food paraffin P-1, with which we melted soft paraffins (SPs), waxes (W), and ceresines (C). Joint fusion of the paraffin P-1 with SP, W, and C was realized at temperatures 20°C above the crystallization temperature (T c) of the highest-boiling product. While compiling the binary mixtures with paraffin P-1 we used the following petroleum products: experimental samples of Ozeksuatsk soft paraffins (SP-1) and Romashkinsk oils, industrial samples of Volgograd NPZ C-65, C-80, and C-85 ceresines, and Yaroslav NPZ protective wax. The chemical compositions and physicochemical characteristics of these petroleum products are given in [7].
The dependences of the phase transformation temperatures T pt (crystallization T c, setting (melting) T m, and H → R transition in the solid state) on the composition of the binary paraffin–wax and paraffin–ceresine composites are presented in the form of “property–composition” phase diagrams.
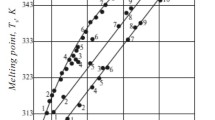
Melting Point. Figure 1 shows the melting point diagram of the paraffin compositions of grade P-1 hard paraffin with SP, petroleum wax ZW-1, and ceresine against “melting point Tm– composition” coordinates.
As seen from Fig. 1, the solubility of the investigated petroleum modifiers in paraffin P-1 increases in the order: C-85, C-80, C-65, ZW-1, SP-2, SP-1. Here, the C-65, C-80, and C-85 modifiers, which are higher than hard paraffin P-1 in melting point, raise the melting points of the compositions. The T m value of the mixture increases with increase in the content of the more high-molecular component, while the degree of decrease of T m here decreases. The largest increase in melting point occurs with additions of high-melting paraffins up to 20 wt. % (curves 5 and 6). The soft paraffins SP-1 and SP-2 reduce the T m value of the composites (curves 1 and 2). ZW-1, which has a T m close to P-1, has little effect on T m of the paraffin–wax compositions.
All the investigated paraffin petroleum products mix well with each other at temperature T ≥ 363 K. The difference between the temperatures at the beginning of crystallization T c and setting or melting T m of both paraffins and their composites with ceresines and petroleum waxes amounted to no more than 0.5-2.5 K. This indicates spontaneous massive crystallization accompanied by three-dimensional structurization of the given dispersed systems.
To identify the factors that affect the value of the melting points T m of the paraffin composites the experimental T m values were compared with the values calculated by the additivity rule and determined from the “melting point T m– average molecular mass M av” relationship [7] on the basis of the calculated values of the average molecular masses of the composites and their CO hydrocarbons.
The T m values of the investigated paraffin compositions were calculated according to the additivity rule by means of the equation:
The calculation showed (Table 1, column 3) that T m of the mixtures of P-1 with SP-1, SP-2, and C-65 obey the additivity rule and agree with the experimental data (column 2). The calculated T m values of the composites of P-1 with C-80 had significant differences from the experimental values (column 3 in comparison with curve 2 for C-80). The results made it possible to conclude that the melting points (Tm) of the mixtures of paraffins with each other and with ceresines obey the additivity rule if the difference between T m values for the mixed components is not greater than 15°C. In order to obtain evidence for and investigate the role of nalkanes in three-dimensional structure formation (solidification) of paraffin compositions (PC) according to the graph for T m = f(M av) the correspondence of the melting point T m of each PC both to its average molecular mass M av and to the molecular masses of its component n-hydrocarbons forming a complex with carbamide (M co) was checked. The graph for T m = f(M av) was presented in [7]. The M av and M co values were calculated by means of the equations:
where M p and M C are the average molecular masses of the paraffin and ceresine respectively, g/mole; M co.p and M co.C are the average molecular masses of the n-hydrocarbons of the paraffin (P) and ceresine (C) respectively forming the complex with carbamide (CO), g/mole; c p and c ts are the concentrations of the paraffin and the ceresine in their mixture, wt. %.
Comparison of the experimental values of the melting point T m of the paraffin–ceresine composites with those determined from the T m—M av graph using the calculated values for the average molecular masses of the composites and their complex-forming KO hydrocarbons is shown in Table 1 (columns 4, 5, and 6). As found, the experimental values of the melting point T m (column 2) for all the mixtures of P-1 with C-65 and the soft paraffins (SPs) agree fairly accurately with the T m values determined for the individual n-alkanes according to curve 1 of the T m—M av graph [7] on the basis of the molecular masses of the KO hydrocarbons of the mixtures calculated by means of Eq. (3) (curve 4). From these experimental and calculated data it follows that the values of the melting temperatures of the complex paraffin and paraffin–ceresine mixtures are determined primarily by the melting temperatures of the n-alkanes present in their compositions. For the mixtures of the P-1 paraffin with the more high-molecular and high-melting ceresines C-80 and C-85 (when the difference in T m of the alloyed petroleum products amounts to 25-30°C with Δn c = 17-20 atoms) their Tm experimental values (Table 1, column 2) prove closer to the values calculated from the average molecular masses (M av) (column 5) and not from the molecular masses of their KO hydrocarbons (M KO) (column 4). In this case, however, as seen from comparison of columns 5 and 6 in Table 1 T m is also determined correctly from the calculated M av, as previously, by the graphical scheme T m = f(M av) for the individual n-alkanes, using curve I and not II [7]. Here, in the case of the existing discrepancies between the experimental and calculated T m values of the composites the calculated values are always lower (column 6 in comparison with column 2 in Table 1). This fact indicates almost equal participation of the hydrocarbons of high-molecular ceresines at the initial stage of three-dimensional structure formation with the paraffins. This may be due to structural characteristics of the molecules of the hydrocarbons in the investigated samples of high-molecular ceresines: the degree of branching in the molecules of the hydrocarbons of C-80 and C-85, as shown in [7], is significantly lower.
By virtue of the practical significance of the melting point of the paraffin composites as an operational characteristic of their quality, mathematical relationships between the melting point of the PC based on P-1 and the content of modifiers were established by stepwise multiple regression with the use of a computer (Table 2). The obtained functional relationships make it possible with an accuracy of up to 95-100% to estimate the values of the melting points for the petroleum paraffin compositions of P-1 with SP-1, SP-2, PW-1, C-65, C-80, and C-85 and to determine the quality and quantity composition of the PC for which the given values of T m can be guaranteed.
Temperatures of the Phase Transformations in the Solid State. The diagrams of the phase states of the investigated PCs are presented in Figs. 2-5.
As shown by the dilatometric investigations and DTA, supported by thermo x-ray diffraction, during cooling the composites of paraffin with the petroleum ceresines (C) are characterized by fractional crystallization first of the high-melting ceresine in the R(α) system, then of the paraffin in the H(α) system at the respective temperatures, and then in the R(α) system (Figs. 4 and 5). The hard and soft paraffins and also the wax ZW-1 crystallize initially in the hexagonal H(Ϭ) and then in the rhombic R(α) phase (Figs. 2 and 3), and the ceresines only crystallize in the R(α) system (Figs. 4 and 5). The temperature of the modification H → R phase transition of the paraffin P-1 in its solid composites with ceresines, irrespective of their quantitative ratio, remains practically constant (308-310 K), which is characteristic of P-1 itself. In the solid state under room conditions (293 K) the binary mixtures of the paraffin (P) with ceresines, waxes, and also soft paraffins (SP) represent heterogeneous systems consisting of two crystalline phases in the R system: R (P) + R (C); R (P) + R (W); R (P) + R(SP).
In one case an isomorphous system is formed during joint crystallization of the hard and soft paraffins in a hexagonal H phase. As shown by the x-ray investigations, limited dissolution of the lower-molecular part of the n-alkanes of ceresine C-65 and the wax ZW-1 in the paraffin P-1 is possible, leading to a small increase of the R parameter of the paraffin crystal lattice. Thus, in the P-1 + C-65 (1:1) composite at 298 K the rhombic phase of the paraffin has the higher value of n c ≈ 28-29 compared with n c ≈ 27 for pure P-1. During crystallization the soft paraffin together with the hard paraffin forms initially an isomorphous hexagonal H phase, and then with decrease of the temperature to 309 K the crystals of paraffin P-1 undergo a hexagonal–rhombic H → R transformation, as a result of which the composite becomes two-phased and remains so after the H → R transition of the soft paraffin.
Thus, by blending the paraffin P-1 with other products it is possible to lower the melting point if this parameter of the added substance is lower than the paraffin and vice versa. Here the melting points of the mixtures of paraffin with ceresines obey the additivity rule if the difference in the melting points of the mixed components is not greater than 15 degrees.
It was established that the composites of paraffin P-1 with ceresines C-65 and C-80 are characterized by separate crystallization first of the high-melting and then of the low-melting components in the R or H systems that are characteristic of them at the given temperatures. The hard (P-1) and soft (SP) paraffins and also the protective wax ZW crystallize first in the H system and then in the R system. The T H → R value of the paraffin P-1 (309 K) hardly changes at all with the addition of ceresine. At 293 K the binary composites of paraffin P-1 with C-65, C-80, and the wax ZW-1 are heterogeneous systems consisting of two phase of the rhombic R system.
References
A. N. Pereverzev, N. F. Bogdanov, Yu. N. Roshchin, Production of Paraffins [in Russian], Khimiya, Moscow (1973), 224 pp.
A. S. Abubakarova, Zh. T. Khadisova, E. A. Aleksandrova, et al., Khim. Tekhnol. Topliv i Masel, No. 2, 38–41 (2014).
S. K. Filatov, Crystal Chemistry and Typomorphism of Minerals [in Russian], Nauka, Leningrad (1985), 56 pp.
S. K. Filatov, Å. N. Kotelnikoya, E. A. Aleksandrova, Zeitschrift für Kristallographie, New Crystal Structures, 172, 35–43 (1985).
Zh. T. Khadisova, A. S. Abubakarova, E. A. Aleksandrova, et al., Khim. Tekhnol. Topliv i Masel, No. 4, 30–33 (2013).
Zh. T. Khadisova, E. A. Aleksandrova, G. M Naumova, Khim. Tekhnol. Topliv i Masel, No. 1, 47–48 (2005).
E. A. Aleksandrova, B. E. Krasavtsev, A. S. Abubakarova, et al., No. 6, 35–39 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 3, pp. 43 – 47, May – June, 2017.
Rights and permissions
About this article
Cite this article
Krasavtsev, B.E., Aleksandrova, E.A., Khadisova, Z.T. et al. Thermal Properties of Wax Compositions. Chem Technol Fuels Oils 53, 360–368 (2017). https://doi.org/10.1007/s10553-017-0812-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-017-0812-8