The heat-treatment mechanisms of superviscous oil in the presence of naturally occurring catalysts (carbonate rock, alumina, etc.) and carboxylic acid at 290-360εC and 1-1.4 MPa pressure are studied. Analysis of the component composition of the transformed oil indicates the predominance of polycondensation reactions over cracking reactions. Based on IR spectroscopic data, the products are characterized, vis-à-vis the original oil, by reduced content of branched structures and sulfoxide groups. The transformed oil samples differ in the temperature at which the viscosity anomaly index more than doubles. The viscosity of the conversion product can be higher or lower than that of the original oil, depending on the process temperature and the type of the mineral catalytic additive.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Deposits of high-viscosity oil and natural bitumen in Russia comprise not only terrigenous but also carbonate reservoirs. These reservoirs have different porous structures and fluid properties [1–3]. Carbonate reservoirs are characterized by low initial strata temperatures, low and middle depths, and low gas factors. Steam-thermal treatment of well bottom-hole zones and pumping heat exchangers into strata are the most widely used methods for recovering heavy oil and natural bitumen. Strata can be heated to 200°C and higher [4]. The extent of polycondensation of oil hydrocarbons that form carbonized substances that plug pores during steam-thermal treatment of the strata increases significantly with increasing temperature and pressure.
Methods for initiating low-temperature oxidation of oil in strata using homogeneous or heterogeneous catalysts were described [5, 6]. A synergic effect can be expected from pumping a steam-air mixture into the strata as a result of combined thermal, gas, and hydrodynamic effects. This is an important prerequisite for the feasibility of research in this area.
The present work focused on evaluating the effects of trace elements in oil-bearing carbonate rock on the transformation of bituminous oil during steam-thermal treatment in the strata.
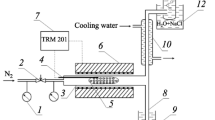
Experiments were conducted in a 200-mL stainless-steel pulsed laboratory reactor. We studied heavy oil from Ashal’chinsk deposit of density 971.5 kg/m3, viscosity 2.771 Pa⋅s at 20°C, and sulfur (S) content 3.9 wt. %. The oil was recovered from depths up to 110 m from the surface where the starting stratum temperature was 8°C; pressure, 0.44 MPa [7]. The minerals added to the carbonate rock were primarily dolomite and calcite. It is noteworthy that clayey minerals containing Al2O3 and having significant catalytic activity were found practically everywhere in the carbonate reservoirs. Roughly dispersed (up to 25 μm) Al2O3 was added to the oil in experiment 2.
It seemed interesting to use steam-air mixtures with a small amount of organic acids during steamthermal treatment in order to improve the filtration-capacity properties of the carbonate rock [3]. The selected organic acid was citric acid. A reaction mixture (oil, mineral additive, water, acid) of total volume 150 mL was loaded into the reactor. The initial pressure was atmospheric and increased to 1-1.4 MPa after heating to 290-360°C. Table 1 presents the experimental thermobaric conditions and the component composition of the obtained transformed oils. The reactor was held at the temperature indicated in Table 1 for 2.5 h.
The studies on the transformation of high-viscosity oil in the presence of mineral additives showed that the oil component composition changed considerably under the thermobaric conditions characteristic of steam-thermal methods (Table 1). The hydrocarbon content decreased at the expense of increasing the resin content in all experiments regardless of the temperature and pressure. The oil component composition in experiment 1 remained practically unchanged. It decreased in experiment 2 by 28% relative to the asphaltene content. The content of benzene resins in the final product increased by 23%, mainly due to a reduced hydrocarbon content, in experiment 3 with the temperature increased to 360°C in the presence of a carbonate additive and carboxylic acid. The formation of coke-like products that precipitated from the liquid phase onto the reactor walls was noted during conversion of heavy oil in the presence of hematite under similar conditions [8]. Carbonized substances were not found experimentally in the final products. An analysis of the product component composition indicated that polycondensation reactions prevailed over cracking reactions.
The structural composition of the transformed oil was studied using IR spectroscopy [8]. IR spectra exhibited characteristic absorption bands for aliphatic structures at 1380 and 1465 cm–1; for methylene chains, 720; for aromatic structures, 1600; for sulfoxides, 1030; and for carboxylate esters, 1740. The contents of isoprenoid structures and sulfoxides decreased during thermal transformation of the oil. This was evident in the decreased spectral parameters for branching C 3 and sourness C 5 (Table 2) that averaged 46% and 25%, respectively.
Experiment 2 gave the most interesting changes. The content of aromatic structures decreased by 5%. This agreed with the component analysis, namely, the reduced contents of asphaltenes with an increased resin content (Table 1). The increase of aromaticity was more pronounced in the presence of only the carbonate additive in experiment 1. This was indicated by the significant strengthening of the absorption band at 1600 cm–1 and spectral parameter C 1. An analysis of trends in the structural changes and component compositions of the obtained oils indicated that the carbonate additive had low catalytic activity in thermally destructive hydrothermal reactions. The situation changed if Al2O3 and carboxylic acid were added to the reaction mixture.
Rheological studies used a cone—plane system at shear rates from 3 to 1312 s–1 and temperatures from 10 to 80°C. The structural viscosity η max (Fig. 1a), Newtonian viscosity η min (Fig. 1b), and viscosity anomaly index θ = η max/η min (Fig. 2) were determined from the flow curves at a given temperature. The last reflected the stability of the structures to shear deformations. The structural viscosity of the samples was related to the contents of resins and asphaltenes that were capable of forming three-dimensional coagulated structures. Such a structure in the dispersed oil could not be viewed as a rigid three-dimensional framework because of the instantaneous generation and destruction of aggregates, the strength of which depended on the balance of forces acting on the system.
The effective viscosity at shear rate 900 s–1, i.e., the region of Newtonian flow, decreased over the whole studied temperature range for samples of transformed oil. The product of experiment 2 was characterized by increased viscosity in both the structural and Newtonian regions and higher shear stress, especially at low temperatures. This could be explained by the accumulation of resins in the final product as a result of oxidation. Thus, intermolecular interactions increased and larger hydrodynamic particles than in the starting oil formed. The viscosity decreased most characteristically for experiment 1 (by 55% at 10°C). Such a change of the flow parameters of the product from experiment 1 could indicate the formation of a dispersed oil system with a supramolecular structure that was more compact than for the starting oil. This created less resistance to liquid movement.
The product of experiment 3 had the lowest structural viscosity. However, its Newtonian viscosity was greater than that of the starting oil. It is noteworthy that the temperatures at which the viscosity anomaly index θ more than doubled differed for the transformed oil. The sharp increase of θ indicated that the oil became less resistant to shear deformations. This was due to the presence of three-dimensional coagulation-crystallization structure in the sample. The differences in the temperatures at which the viscosity anomaly maxima were observed were related to qualitative and quantitative changes in the oil paraffinic hydrocarbons.
Conclusions
-
transformation of bituminous oil under the thermobaric conditions of experiment 1 in the presence of a carbonate additive decreased the oil aliphaticity and the content of branched structures in it and more than halved the viscosity. The component composition remained practically unchanged;
-
the lower process temperature in experiment 2 led to resin formation. However, the presence of Al2O3 in the reaction mixture led to cracking of asphaltenes, reduced the oil aromaticity and sourness, and increased its viscosity;
-
adding a carboxylic acid to the reaction mixture in experiment 3 at a temperature close to that of experiment 1 led to formation of benzene resins, increased the aromaticity, decreased the structural viscosity, and reduced the resistance to shear deformations at 20°C by 88%.
References
R. Kh. Muslimov, G. V. Romanov, G. P. Kayukova, et al., Neft2. Gaz. Novatsii, No. 2, 21-29 (2012).
R. S. Khisamov, N. S. Gatiyatullin, I. E. Shargorodskii, et al., Geology and Development of Natural Bitumen Deposits of the Republic of Tatarstan [in Russian], Fen, Kazan, 2007, 295 pp.
R. Kh. Muslimov, G. V. Romanov, G. P. Kayukova, et al., Complex Development of Heavy Oils and Natural Bitumens of the Perm System of the Republic of Tatarstan [in Russian], Fen, Kazan, 2012, 396 pp.
V. A. Lyubimenko, N. N. Petrukhina, B. P. Tumanyan, et al., Khim. Tekhnol. Topl. Masel, No. 4, 27-33 (2012).
R. Hashemi and P. Pereira-Almao, Canadian Unconventional Resources Conference, Nov. 15-17, 2011, Alberta, Canada; SPE-149257-MS.
W. Qin and Z. Xiao, Adv. Mater. Res., 608-609, 1428-1432 (2013).
S. M. Petrov, D. A. Khalikova, Ya. I. I. Abdelsalam, et al., Vestn. Kazan. Tekhnol. Univ., No. 18, 261-265 (2013).
I. M. Abdrafikova, A. I. Ramazanova, G. P. Kayukova, et al., Vestn. Kazan. Tekhnol. Univ., No. 6, 237-242 (2013).
Acknowledgments
The work was sponsored by a subsidy for state support of Kazan Federal University to increase its competitiveness among leading global science-education centers.
The work was performed using a subsidy to Kazan Federal University for performing a state task on scientific activity.
The work was sponsored by a grant of the Russian Federation President MK-2054.2014.5.
Author information
Authors and Affiliations
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 1, pp. 79 – 82, January– February, 2015.
Rights and permissions
About this article
Cite this article
Petrov, S.M., Abdelsalam, Y.I.I., Vakhin, A.V. et al. Study of the Rheological Properties of Heat-Treatment Products of Asphaltic Oils in the Presence of Rock-Forming Minerals. Chem Technol Fuels Oils 51, 133–139 (2015). https://doi.org/10.1007/s10553-015-0585-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-015-0585-x