This paper is devoted to a study of the characteristic features of the composition and structure of crude oils from terrigenous Devonian producing deposits in the Minnibaevskii section of the Romashkino and Pervomai fields. Using the SynOil program, we identified the features of the molecular weight distribution of n-paraffins, cyclohexanes, and monoalkylbenzenes associated with the formation and transformation conditions in the crude oil deposits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Minnibaevskii section is one of the central areas of the Romashkino oil field. It is confined to the western and central parts of the South-Tatar arch. The principal developed features are producing sands of the Kynovian (D0) and Pashiiskii (D1) horizons at average depths of 1750–1770 m. The Minnibaevskii section is one of several highly productive oil-bearing sections of the Romashkino field. Crude oil has been recovered from it since 1950. The Pervomai field is located within the Pervomai-Bondyuzh embankment on the eastern slope of the North-Tatar arch. It was put into operation in 1962 and is in its final stage with an output 92.2% of the initial recoverable reserves. The principal oil-bearing features are the Upper Devonian Timansk-Pashiiskii deposits [1].
Geochemical parameters based on the composition of aliphatic and aromatic hydrocarbons are traditionally used to establish the composition and structure of crude oils. They can answer several important questions regarding the solution of engineering and geological problems. However, some of the questions remain unanswered. In particular, both the formation stage of oil and gas deposits and their preservation conditions in nature are unresolved [2–5]. A new methodical approach and set of required parameters are required in order to solve these problems. In the present work, one solution to these problems that examines the molecular-weight distribution (MWD) of aliphatic and aromatic C12+ hydrocarbons is presented using crude oils from the Minnibaevskii section of the Romashkino and Pervomai fields as examples.
The crude oils were studied in the Laboratory of Fossil Fuel Geochemistry, Kazan Federal University. They were separated into oils, resins, and asphaltenes. The oil fraction was obtained by liquid-adsorption chromatography on silica gel. Hydrocarbons in the oil fraction were investigated using GC/MS on a Turbo Mass Gold instrument (PerkinElmer) and computer data processing in SIM mode for ions with m/z = 85 for n-paraffins, m/z = 83 for cyclohexanes, m/z = 92 for monoalkylbenzenes, and m/z = 134 for trimethylalkylbenzenes. We used a capillary column (30 m × 0.32 mm) with PE-XLB stationary phase. The chromatography temperature was programmed from 100 to 300°C at 12.5°C/min for 100-150°C and 3°C/min for 150-300°C. The initial temperature was held constant for 2 min; the final temperature (300°C), for 14 min. The vaporizer temperature was 300°C. The carrier gas was He at flow rate 2 mL/min.
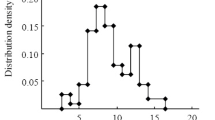
Figure 1 shows the MWD of C11-C30 n-paraffins in crude oils of Minnibaevskii section of Romashkino and Pervomai fields. Members of the series were distributed non-uniformly in two ranges with certain regularities. The first range comprised C14-C16 n-paraffins and showed a sharp increase by two times and more for the last member of the series relative to the first two. Starting with C16+, a second range was characterized by a smooth decrease of the succeeding member of the series relative to the preceding one. This distribution of n-paraffins was characteristic of the main part of crude oils from the Romashkino and Pervomai fields. The homologous series of n-paraffins for the main part of the studied crude oils started from C14. n-Paraffins up to C14 were missing because the light part of these oils was removed. This was consistent with their initial boiling point of 180-200°C.
The examination of this group of crude oils paid special attention to oil sample 890 with a different n-paraffin MWD. The difference consisted of a smooth reduction of the contents of series members with an increasing number of C atoms starting with C12. Thus, C12-C14 hydrocarbons included a significant fraction, in contrast with the other crude oils. The occurrence of crude oil with such a MWD of n-paraffins in the field was interesting and requires research to establish the relationship of this crude oil and the others.
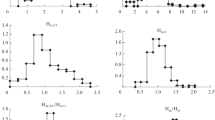
The SynOil program was used to estimate the composition of crude oil 890. It could establish the nature of the MWD for n-paraffins and other compounds forming homologous series of varying composition [6, 7]. Figure 2 plots results from a simulated separation. The employed method is described below in detail.
Certain trends were noted in the MWD of C11-C30 n-paraffins. Firstly, portions of the homolog distribution did and did not obey a mathematical equation. Thus, a portion representing C12-C18 hydrocarbons with a smooth reduction of their content with increasing number of C atoms was observed for crude oil 890. It obeyed the exponential equation
where A and B are coefficients; C, the number of C atoms in the molecule; and N, the n-paraffin content.
Figure 2a shows this as the complete coincidence of the MWD curve in the C13-C18 range with the standard curve corresponding to the exponential equation for this series. However, a deviation from the standard curve was observed starting with C19. The distribution was different for C19+ n-alkanes and did not obey the described exponential equation. Thus, two portions reflecting crude-oil formation processes were found in the homologous series of n-paraffins by modeling the composition of crude oil 890.
Other groups of hydrocarbons with a broad composition of homologous series could be used to study crude-oil formation and reformation processes. This applied firstly to alkylcyclohexanes and alkylbenzenes. Figure 2b shows results from a separation of high-molecular-weight alkylcyclohexanes (ACH) and alkylbenzenes (AB) in crude oil 890 that was simulated using the SynOil program. The obtained MWD curves were similar to the distribution of n-paraffins in this crude oil. This consisted of separate curve portions that obeyed an exponential equation and portions that deviated from the standard curve. Thus, all examined homologous series of hydrocarbons had the same type of MWD.
The natural conditions under which the aliphatic and cyclic compounds originated and transformed had to be considered in examining their MWD features. This was due to the fact that cyclic compounds like those in crude oils are practically unknown in nature. They occur in crude oil because of several complicated transformation processes of the starting organic matter [8, 9]. These processes in deposits cannot always give a broad series of homologous products or a MWD of compounds that is amenable to a mathematical description. Thus, it is especially necessary to note that identical MWD features were observed in their homologous series despite the different formation mechanisms of the aliphatic and cyclic hydrocarbons.
Mass-exchange processes between the gas and liquid phases in the deposits were indicated by analyzing the observed exponential distribution of members in the series of n-paraffins, ACH, and AB. A MWD of this nature could not be produced by any catalytic or thermal process on the organic matter in the deposits. Mass-exchange processes involve a large volume of gas. Under these conditions, compounds in the liquid phase under non-equilibrium conditions transfer to the gas phase and form an ordered distribution of series members. As a rule, n-paraffins (and cyclic hydrocarbons) in the gas phase have a MWD that decays exponentially with increasing molecular weight and obeys the formula given above. Such a MWD is typical exclusively of gas condensates, for which the MWD of n-paraffins coincides with the standard curve. Therefore, the presence of the line of standard values that coincided partially or fully with the MWD curve indicated that the crude oil included a condensate component; the observed deviation, a crude-oil or bitumen component. Therefore, the sample was a hydrocarbon mixture of compounds of different genesis, generation conditions, or deposit formation stage.
Thus, the simulated separation of crude oil 890 indicated that it was a condensate including 5.1% heavy hydrocarbons. It should be noted that the condensate and crude-oil components had different natures.
The MWD of the other crude oils were established analogously using the SynOil program. Figure 3 shows the MWD of n-paraffins, ACH, and AB in crude oil 159 as examples. It can be seen that the two portions that were described previously for crude oil 890 were clearly present in all distributions of homologous series members. The range C15-C18 had a smoothly decaying distribution of series members with increasing numbers of C atoms that obeyed the exponential equation. The other portion in all series of C19-C30 hydrocarbons deviated from the ideal MWD, which was a continuation of the hydrocarbon distribution of the preceding portion. The C19-C30 range in each series comprised 30-40% of the total hydrocarbon content of the given series. Keeping in mind the identical hydrocarbon contents in the deviation range of all series, it could be assumed that this content was common for all crude oils. Thus, these crude oils were also composed of 60-70% condensate and 30-40% heavy-oil.
Common signatures in the studied homologous series of n-paraffins, ACH, and AB were considered in examining the genetic relationship among the examined crude oils. First, the AB must be noted. The MWD of AB in the range C18-C30 for all crude oils were identical (Fig. 2c and 3c). AB C21 and C23, which are derivatives of natural aromatic compounds, were identified in this range in all crude oils.
Another group of monoaromatic hydrocarbons C10-C40 must be noted among the relict aromatic compounds [10, 11]. These are products of isorenieratane (C40H60) destruction in the deposits with the structure

This compound is a relict that preserved all principal signatures of the biological compound isorenieratane [11]. Its hydrocarbon chain was destroyed in the deposits during thermocatalytic transformations to form AB C10-C32. Figure 4 shows mass fragmentation patterns using the ion with m/z = 134 that reflected the presence of tetraalkylbenzenes in crude oils 890 and 159. Their low-molecular-weight regions and MWD were different. Thus, a complete set of these compounds starting with C10 was observed for crude oil 890 whereas the homologous series began with C13 for crude oil 159. Considering that the whole set of compounds was formed during transformation of the starting matter, the absence of some of them in crude oil 159 was most likely related to the storage conditions (poor ability of the covering horizon to retain light hydrocarbons) of the crude oil deposit adjoining the well section.
Thus, the study of crude oils from Romashkino and Pervomai fields using simulated separation of n-paraffins, ACH, and AB according to the SynOil program showed that the crude oils were formed under complicated conditions. All crude oils were based on a condensate component, which was well represented in crude oil 890, and a crude-oil component that differed with the formation conditions. The presence or absence of one group of low-molecular-weight compounds or another in the crude oils was indicative of their natural storage conditions.
In conclusion, it is noteworthy that the SynOil program was capable of solving problems related to estimating the MWD of members of large homologous series of compounds. The methodical approach used in this program was based on a mathematical description of the distribution of C10-C30 n-paraffins and could identify highly accurately all features in the behavior of the series members, as was done in the present work.
References
R. Kh. Muslimov (ed.), Republic of Tatarstan Oil and Gas Reserves. Geology and Development of Oil Fields [in Russian], Fen AN RT, Kazan, 2007, Vol. 1, p. 316; Vol. 2, p. 524.
G. P. Kayukova, G. V. Romanov, and I. N. Plotnikova, Georesursy, 47, 37–40 (2012).
G. P. Kayukova, G. V. Romanov, R. G. Luk’yanova, et al., Organic Geochemistry of Sedimentary Strata and Base Rock of Tatarstan [in Russian], GEOS, Moscow, 2009, 487 pp.
G. P. Kayukova, I. P. Kosachev, I. N. Plotnikova, et al., Geol. Nefti Gaza, No. 3, 53–64 (2014).
I. N. Plotnikova, Int. J. Earth Sci., 97, 1131–1142 (2008).
S. B. Ostroukhov, Vestn. VolgGASU, Ser.: Tekh. Nauki, No. 6, 198–203 (2006).
S. B. Ostroukhov, A. S. Ostroukhova, and V. I. Petrenko, in: Collection of Articles of the Filial of LUKOIL-Engineering VolgogradNIPImorneft OOO, Volgograd, 2011; No. 70: Geology and Development of Fields in Lower Volga Region and Northern Caspian Area [in Russian], pp. 206–215.
S. B. Ostroukhov, O. A. Aref’ev, S. D. Pustil’nikova, et al., Neftekhimiya, 23, No. 1, 20–30 (1983).
S. B. Ostroukhov, in: Proceedings of the IVth International Conference “Chemistry of Oil and Gas” [in Russian], Tomsk, 2000, Vol. 1, pp. 349–354.
S. B. Ostroukhov, O. A. Aref’ ev, V. M. Makushina, et al., Neftekhimiya, 22, No. 6, 723–728 (1982).
R. E. Summons and T. G. Powell, Geochim. Cosmochim. Acta, 51, 557–566 (1987).
Acknowledgments
The work was performed using a subsidy from Kazan Federal University for the State Task in Scientific Activity and a subsidy for state support of Kazan Federal University for improving its competitiveness among world-leading science-education centers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 6, pp. 70 – 74, November – December, 2014.
Rights and permissions
About this article
Cite this article
Ostroukhov, S.B., Plotnikova, I.N., Nosova, F.F. et al. Characteristic Features of the Composition and Structure of Crude Oils From the Pervomai and Romashkino Fields in Tatarstan. Chem Technol Fuels Oils 50, 561–568 (2015). https://doi.org/10.1007/s10553-015-0564-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-015-0564-2