Abstract
Purpose
We aimed to study the associations between androgen-deprivation therapy (ADT)-induced weight changes and prostate cancer (PC) progression and mortality in men who had undergone radical prostatectomy (RP).
Methods
Data from the Shared Equal Access Regional Cancer Hospital (SEARCH) cohort were used to study the associations between weight change approximately 1-year post-ADT initiation and metastases, castration-resistant prostate cancer (CRPC), all-cause mortality (ACM), and PC-specific mortality (PCSM) in 357 patients who had undergone RP between 1988 and 2014. We estimated hazard ratios (HR) and 95% confidence intervals (95% CI) using covariate-adjusted Cox regression models for associations between weight loss, and weight gains of 2.3 kg or more, and PC progression and mortality post-ADT.
Results
During a median (IQR) follow-up of 81 (46–119) months, 55 men were diagnosed with metastases, 61 with CRPC, 36 died of PC, and 122 died of any cause. In multivariable analysis, weight loss was associated with increases in risks of metastases (HR 3.13; 95% CI 1.40–6.97), PCSM (HR 4.73; 95% CI 1.59–14.0), and ACM (HR 2.16; 95% CI 1.25–3.74) compared with mild weight gains of ≤ 2.2. Results were slightly attenuated but remained statistically significant in analyses that accounted for competing risks of non-PC death. Estimates for the associations between weight gains of ≥ 2.3 kg and metastases (HR 1.58; 95% CI 0.73–3.42), CRPC (HR 1.33; 95% CI 0.66–2.66), and PCSM (HR 2.44; 95% CI 0.84–7.11) were elevated, but not statistically significant.
Conclusions
Our results suggest that weight loss following ADT initiation in men who have undergone RP is a poor prognostic sign. If confirmed in future studies, testing ways to mitigate weight loss post-ADT may be warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the lack of Level I evidence showing benefit, men with prostate cancer (PC) who fail primary therapy are placed on early androgen-deprivation therapy (ADT) as the de facto standard of care. However, there are growing concerns over this strategy owing to the side effects of ADT and importantly since the 5-year survival for PC is now approaching 100% [1]. While numerous side effects have been documented (e.g., fatigue, loss of libido, hot flashes, osteoporosis), there is now strong evidence indicating that ADT also leads to body composition changes, including increases in fat mass and decreases in lean mass and muscle strength [2,3,4,5]. Specifically, during the first year of ADT, fat mass can increase by about 10%, while lean mass can decrease by about 3% [3, 6]. The net result is often weight gain and obesity which may lead to diabetes and cardiovascular disease [7,8,9]. A previous study from the Shared Equal Access Regional Cancer Hospital (SEARCH) database found that men gain on average 2.2 kg during the first year of therapy [10]. This weight gain is in line with reports in the literature; however, weight gains may be greater in younger, non-obese patients [7, 11]. Data from the SEARCH database also indicate that nearly a third of men experienced weight loss on ADT. Given the evidence that obesity at ADT initiation is associated with early development of castrate-resistant prostate cancer (CRPC) [12], an increase in the risk of PC progression might also be expected with ADT-induced weight gain.
To date, studies have not examined the impact of weight gain on PC outcomes such as metastases, CRPC, PC-specific mortality (PCSM), and all-cause mortality (ACM) while undergoing ADT. Therefore, we sought to investigate the associations between weight change, estimated from weights documented pre- and post-ADT initiation, and PC outcomes in men who had undergone radical prostatectomy (RP). Data were obtained from the SEARCH database [13]. We hypothesized that greater weight gain would be associated with worse oncological outcomes.
Materials and methods
Study population
Approval was obtained from the Veterans Affairs Institutional Review Board. SEARCH is a retrospective cohort of men undergoing RP from 1988 to 2014 and followed up through 2016 at six Veterans Affairs Hospitals (Palo Alto, West Los Angeles, and San Diego, CA; Durham and Asheville, NC; Augusta, GA). Detailed data pertaining to demographic, clinical, and pathological factors were extracted from hospital charts and included in the SEARCH database [14]. Patients who had received neoadjuvant androgen-deprivation or radiation therapy were not included in SEARCH.
Pre-ADT weight was defined as weight measurement closest to, but within 12 months prior to ADT initiation. Post-ADT weight was defined as the weight measurement closest to 12 months, but within 6–18 months post-ADT initiation. Height was taken as the median of all height measurements and assumed to be constant over time. Body mass index (BMI) prior to ADT initiation was calculated as weight in kilograms divided by height in meters squared. Absolute weight change from pre-ADT to post-ADT was categorized into three groups: weight loss if post-ADT weight was less than pre-ADT weight, stable and mild weight gain if weight did not change or increased 0–2.2 kg, and moderate-to-severe weight gain if weight increased ≥ 2.3 kg. We used 2.3 kg (~ 5 pounds) as the cut-point for weight gain since this was the median weight gain observed after ADT initiation in a prior study from the SEARCH database [10]. The difference in pre-ADT weight and the weight closest to 12 months prior to the pre-ADT weight (± 6 months) was used to determine the weight change trajectory prior to ADT initiation to facilitate interpretation of post-ADT weight changes.
In sensitivity analysis, we established weight change categories by ranking men according to absolute weight change (e.g., loss to gain post-ADT), and dividing the men into tertiles with corresponding tertile cut-points of weight change used to create weight change groups.
CRPC was defined as a PSA rise of ≥ 2 ng/mL and ≥ 25% from the post-ADT nadir while being castrate, defined as serum testosterone levels < 50 ng/dL, bilateral orchiectomy, or continuous receipt of luteinizing hormone releasing hormone agonist or antagonist. Development of metastases was determined radiographically as evidence of prostate cancer outside of the prostate, seminal vesicles, or pelvic lymph nodes. PCSM was comprised of metastatic progressive CRPC at time of death with no obvious indication of other causes of death and ACM was comprised of death from any cause. Mortality data for the SEARCH database were manually abstracted from review of the VA electronic health records and cross-checked against the National Death Index in cases where data were flagged as incomplete.
Of 5,515 men identified, we excluded men who had never received ADT (n = 4,535), had metastases prior to ADT initiation (n = 108), did not have pre-ADT and post-ADT weights documented (n = 409), or had missing information on covariates (n = 76). Treatment with ADT was at the discretion of the attending physician and was either given adjuvantly or for biochemical recurrence. To limit the effect of existing advanced progression of PC at the time of ADT initiation, we excluded men who had developed metastases or CRPC within 18 months of ADT initiation (n = 28). In addition, men with weight changes (loss or gain) greater than three standard deviations from the mean were excluded as outliers (n = 2), resulting in a final study cohort of 357 men (Fig. 1). Characteristics of men included in the study vs. those excluded were compared to assess differences between groups.
Statistical methods
PSA doubling time (PSADT) leading up to ADT initiation was calculated by log(2) divided by the slope of the linear regression of log(PSA) over time in months. Subjects with PSADT < 0 or > 120 were considered to have very slow PSA rise and were assigned to 120 months. All PSA values 2 years prior to ADT initiation but after RP and radiation therapy, if applicable, were used to calculate PSADT. This calculation required at least two PSA values over at least 3 months. Kruskal–Wallis and Chi-square tests were used to compare differences between group medians for non-normally distributed variables and proportions, respectively.
Cox proportional hazards models were used to test the association between weight change after ADT and the risk of metastases, CRPC, ACM, and PCSM. A landmark of 18 months after ADT initiation was established since patients could have had post-ADT initiation weight measurements collected up until 18 months. Absolute weight change, as previously employed in studies examining the relation between obesity and PC [11, 15], was the main exposure and defined as a categorical variable as described above with the reference group comprising of men with stable and mild weight gain (≤ 2.2 kg). Age and BMI (pre-ADT), known biological confounders, identified a priori were forced into all regression models as continuous variables, regardless of impact on the association between weight status and outcome. Other candidate covariates identified a priori were evaluated and included in the models if their inclusion resulted in at least a 10% change in the main exposure (weight change) point-estimate (change-in-estimate approach) [16]. Candidate covariates included year of ADT initiation (continuous), race (Black vs. non-Black), PSA at ADT initiation (continuous), pre-ADT PSADT (< 9 months vs. ≥9 months vs. unknown), pathological grade group (1 vs. 2–3 vs. 4–5) [17], positive surgical margins (yes vs. no), extracapsular extension (yes vs. no), seminal vesicle invasion (yes vs. no), positive lymph nodes (yes vs. no vs. not done) having received adjuvant radiation therapy at any time (yes vs. no), and time from RP surgery to ADT. Cox regression models were tested for the assumption of proportional hazards using Schoenfeld residuals. We assessed collinearity of variables using the variance inflation factor (VIF). Kaplan–Meier curves were used to plot the relationship between weight change and each outcome. Time-to-event differences among weight change groups were tested using the log-rank test.
In a secondary analysis, we repeated the analyses above in models where non-PCSM was accounted for as a competing risk. In addition, we tested whether adjusting for the time between pre- and post-ADT weight measurements affected the results. To examine robustness of our results, we conducted sensitivity analyses. First, to assess the impact of different weight change categories, we re-analyzed the data using cut-points for groups corresponding to tertiles. Second, to assess the impact of the time-windows for capturing pre- and post-ADT weights, we used stricter definitions for cut-off boundaries. Weight between 0 and 3 months before ADT initiation was used for pre-ADT weight and weight between 9 and 15 months post-ADT was used for 1-year post-ADT weight. Third, to minimize the inclusion of undiagnosed more advanced cancer, we extended the ‘landmark date’ from 18 to 24 months post-ADT. Statistical analyses were performed using STATA 13.0 (Stata Corp., College Station, Texas).
Results
One hundred and thirteen (32%) men lost weight after ADT initiation, 72 (20%) experienced mild weight gain, and 172 (48%) experienced moderate-to-severe weight gain (Table 1). Median age at ADT initiation was 65 years (IQR 60–71) and median year of ADT initiation was 2009 (IQR 2006–2012). Overall median time from RP to ADT was 27.2 months (IQR 6.2–69.5), with the time greatest for those who lost weight and least for those who gained weight, [44.1 (IQR 10.6–79.7) vs. 18.3 (IQR 4.6–59.4), respectively, p = 0.002]. For all groups combined, median pre-ADT BMI was 28.1 (IQR 25.4–31.0) with slightly higher BMI observed among men who lost compared with those who gained weight, [29.3 (IQR 26.8–32.5) vs 27.3 (IQR 25.2–30.7), respectively, p = 0.01]. For all groups combined, median post-ADT BMI was unchanged from pre-ADT BMI; however, the moderate-to-severe weight gain group had the highest BMI 29.1 (IQR 26.7–32.67), followed by the weight loss group, 28.1 (IQR 25.1–30.9). Overall median time from pre-ADT weight to ADT initiation was 1.2 months (IQR 0.3–2.3), median time from ADT initiation to post-ADT weight 12.0 months (IQR 10.9–12.7), and 13.0 (IQR 12.0–14.6) months for between weight measurements, with no differences in median times between weight status groups. Overall median pre-ADT weight was 88.5 kg (IQR 79.2–100.3) and post-ADT weight was 90.7 kg (IQR 81.5–102.5). Pre-ADT weights were not statistically significantly different between weight status groups. The trajectory of weight change prior to ADT was determined for men with available weights (n = 325), approximately 12 months [median 12.1 (IQR 11–12.7)] prior to the pre-ADT weights. Pre-ADT weight changes (median; IQR) were 0.88 kg weight gain (− 1.81 to 3.36), 0.18 kg weight gain (− 2.27 to 2.72), and − 0.43 kg weight loss (− 2.99 to 2.31) for weight loss, mild weight gain, and moderate-to-severe weight gain groups, respectively. Differences among groups were statistically significant (Kruskal–Wallis test, p = 0.031).
During a median follow-up of 81 months (Q1, Q3: 45, 121) from ADT initiation, 55 patients developed metastases, 61 developed CRPC, 36 died from PC, and 122 died from any cause. A comparison of characteristics between the 357 men included in this study and the 515 men excluded for missing data (Fig. 1) indicated that the latter group was treated at an earlier time, consistent with medical records being less complete in earlier years (Supplementary Table 1). Clinical factors indicated that these men had somewhat more advanced disease; however, these same clinical factors were well balanced in distribution across study weight status groups.
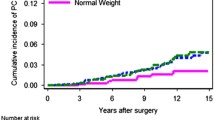
Figure 2 shows the Kaplan–Meier survival curves for weight change categories and each outcome. Log-rank tests for time-to-event differences between weight change groups were null for all outcomes, except for ACM (p = 0.005).
Age, age and BMI-adjusted, and multivariable Cox proportional hazards models are shown in (Table 2). Interaction terms between pre-ADT PSA and log time, and age and log time, were included in CRPC and ACM regression models, respectively, to better meet the assumption of proportional of hazards. Issues with multicollinearity were not found. HRs were elevated but not statistically significant for the associations between ≥ 2.3 kg weight gain and risks of metastases (HR 1.58; 95% CI 0.73–3.42), CRPC (HR 1.33; 95% CI 0.66–2.66), and PCSM (HR 2.44; 95% CI 0.84–7.11) compared with the reference group (stable weight or mild weight gain: 0 to ≤ 2.2 kg). There was no association with ACM (HR 0.97; 95% CI 0.56–1.68). For weight loss, HRs were elevated but not statistically significant in age- and age- and BMI-adjusted models. Multivariable adjusted HRs were elevated and statistically significant for weight loss and metastases (HR 3.13; 95% CI 1.40–6.97), ACM (HR 2.16; 95% CI 1.25–3.74), and PCSM (HR 4.73; 95% CI 1.59–14.0), but not CRPC (HR 1.75; 95% CI 0.83–3.68). Adjustment for time between pre- and post-ADT weight measurements did not modify results (data not shown).
In secondary analyses, we accounted for competing risks of non-PCSM. Results for the association between ≥ 2.3 kg weight gain and outcomes remained null. Weight loss associated with risks of metastases and PCSM were slightly attenuated (HR 2.51; 95% CI 1.11–5.65, and HR 3.52; 95% CI 1.22–11.10, respectively), but remained statistically significant (Supplementary Table 2).
In sensitivity analyses where tertile cut-points for weight change were established at < 0.1 kg, 0.1–3.7 kg (reference group), and ≥ 3.8 kg, for tertiles 1, 2, and 3, respectively, results for weight loss and metastases, ACM, and PCSM were somewhat attenuated in comparison with the main analyses (Supplementary Table 3) but remained elevated and statistically significant. In additional sensitivity analyses, where more stringent cut-offs were used for pre- and post-weights, 83 participants were excluded. Compared with the primary analyses, HRs for weight loss and metastases, ACM, and PCSM increased in magnitude, albeit with reduced precision, but retained statistical significance (Supplementary Table 4). In addition, the HRs for weight loss and CRPC (HR 3.08, 95% CI 1.25–7.60), and moderate-to-severe weight gain and PCSM (HR 4.09, 95% CI 1.12–15.0) increased and became statistically significant. Increasing the landmark date from 18 to 24 months post-ADT had negligible effect on HRs despite the exclusion of 21 participants (data not shown).
Discussion
Much evidence supports the role of obesity in increasing the risk of aggressive PC and PC progression [14, 18,19,20]. For men with recurrent or metastatic disease, ADT is standard of care treatment. While ADT is quite effective at lowering PSA levels, it can result in various metabolic changes including weight gain, which in theory may promote PC progression. We have previously shown that obesity at the time of ADT is linked with increased risk for CRPC [12]. However, no study to date has examined the effect of weight change following ADT initiation and long-term outcomes. We hypothesized that weight gain during the first year following ADT initiation would be associated with an increased risk of metastases, CRPC, and PCSM. At a median follow-up time of 81 months among 357 men starting ADT after RP, our findings did not support this hypothesis. Although HRs for metastases and PCSM were elevated with moderate-to-severe weight gain (≥ 2.3 kg vs. 0 to ≤ 2.2 kg), results did not reach statistical significance in our main analyses. We previously reported, and now confirm with a larger sample size, that about a quarter of the men in the SEARCH database experienced weight loss after ADT initiation [10]. An unanticipated finding in this study was that weight loss was associated with an increase in PC progression with elevated HRs reaching statistical significance for metastases, ACM, and PCSM. Furthermore, in additional analyses that accounted for non-PC death as a competing risk, risks remained elevated and statistically significant. If confirmed in other studies, these results would suggest that weight loss after ADT is a poor prognostic sign, and more importantly highlights the need for additional investigations to determine the underlying mechanisms that link the weight loss with poor outcomes.
While body composition changes due to ADT are well documented and include increases in fat mass and decreases in lean mass, which may lead to sarcopenic obesity [1, 6, 21,22,23], and conceivably weight loss concurrent with increases in fat mass, our study is the first to specifically examine the relation between post-ADT weight change and PC disease progression and mortality. Moreover, our finding that more men in the post-ADT weight loss group gained weight, rather than lost pre-ADT, indicates that weight loss was not an established pre-ADT weight change trajectory. On the contrary these findings strongly suggest that this is not an early sign of cachexia, but an effect of ADT, though of course this requires further validation. Over the longer term, an understanding of the mechanisms involved could lead to targeted interventions that minimize the excess adverse risk associated with weight loss.
Although the pathophysiology and underlying mechanisms are currently poorly understood, evidence suggests that ADT-induced body fat accumulation may not be associated with the same risk for PC progression as that arising from non-ADT linked obesity [7, 24]. For example, following ADT, serum high density lipoprotein levels increase rather than decrease as generally seen with obesity [1, 7]. In addition, with ADT, adipose tissue deposition is predominantly subcutaneous rather than visceral. It is the latter pattern of adiposity that is implicated in the development of cardiometabolic risk factors and subsequent obesity-related diseases [1, 6, 25, 26]. Nonetheless, there are metabolic and pathophysiologic similarities between ADT-induced weight gain and weight gain due to non-ADT linked obesity. These include the development of insulin resistance, hypertriglyceridemia, and diabetes [1, 7,8,9], with some evidence, albeit inconsistent, suggesting subsequent increases in the known obesity-related disorders, such as cardiovascular disease [7, 8].
Considering the major gaps in knowledge with respect to health risks associated with ADT-induced obesity, our null results for weight gain and PC progression and PCSM warrant attention. Weight gain during ADT occurs mainly during the first year of treatment but can continue beyond that time [7, 23]. Hence, men in our reference group (weight stable) could have gained substantial weight beyond our last follow-up time. As such, their risk of disease progression might have more closely resembled men in the moderate-to-severe weight gain group, which could have led to an underestimation of the association between weight gain and outcomes. However, we believe this misclassification was unlikely since men who gain substantial amounts of weight early in treatment are most likely to continue gaining weight in subsequent years [7].
It has also been reported that men who gain weight post-ADT tend to be younger, have a lower BMI and be healthier than men who do not [22]. If true, then baseline risks of disease progression at ADT initiation could favor better outcomes among men gaining weight vs. weight stable men, potentially nullifying the effects of weight gain. In our study, although BMI at ADT was correlated with weight change category, this was driven by higher BMI in the weight loss group. In addition, we did not find that men who gained weight were younger had lower BMI or more favorable clinical characteristics than men in the reference group (weight stable). Moreover, results were unchanged after adjusting for baseline characteristics.
In contrast to the limited follow-up time for the ascertainment of weight status, we studied outcomes over an extended period (median time 81 months). This duration of follow-up is of greater relevance to testing our hypothesis and hence, increases the credibility of our results. A small exploratory study (n = 53) with a median follow-up of 76 months reported findings consistent with our results [23]. Body composition, including total fat mass, was assessed using dual-energy X-ray absorptiometry in men with non-metastatic PC. At 1 and 2 years of ADT, increases in fat mass were not associated with disease progression or recurrence [23].
Given the ongoing uncertainty of health outcomes linked with ADT-induced weight gain, well-designed, large, prospective studies that accurately assess body fat distribution, metabolic effects, and long-term outcomes are needed to better characterize the full spectrum of effects of ADT-induced increase in adiposity. It should be noted, however, that avoidance and/or correction of weight gain may have other health benefits, such as reducing the risk of diabetes [26].
A surprising finding in our study was that men who lost weight were at increased risks of metastases, ACM and PCSM compared with those experiencing mild weight gain. Men diagnosed with metastases or CRPC within 18 months of ADT initiation were excluded from our analysis, hence, we do not believe that these men were exhibiting ‘cancer cachexia’ characteristic of underlying metastatic disease. Furthermore, there was a negligible impact on risk estimates when we excluded men diagnosed within 24 months post-ADT initiation (n = 21) in sensitivity analyses. It is possible that men in the weight loss group experienced body composition changes consistent with severe ADT-induced sarcopenia, although the cause is unclear. It is also unknown if the weight loss was intentional or unintentional. Men in the weight loss group had higher BMI at ADT initiation, and more men within this group had been gaining weight just prior to ADT compared to the other two groups. These men may have intentionally attempted to lose weight and inadvertently used methods that exacerbate lean mass loss. Weight loss driven by loss of lean mass could be an early indicator of poor outcomes and if identified prior to, or early in the administration of ADT, strategies that mitigate its occurrence and severity might be offered to improve long-term outcomes.
The specific effects of ADT on muscle mass have not been elucidated [27]. However, since the loss of lean muscle mass adversely affects overall health [28], research has focused on understanding the relationship between declines in physical function due to muscle loss and the relationship to quality of life (QOL). For example, a study of men with non-metastatic PC on continuous ADT found that ADT associated with physical declines in grip strength, lower extremity function, and endurance, can persist or worsen up to 36 months and be accompanied by reductions in self-reported QOL [29]. To combat muscle loss and declines in physical function, and improve QOL, multiple studies have focused on the effectiveness of lifestyle interventions, such as exercise programs. In a feasibility study of 50 men, Bourke et al. [30] found that men on ADT randomized to a 12-week lifestyle program comprising aerobic and resistance exercise showed improvements in muscle strength. The improvement in strength is congruent with the results of previous resistance and aerobic exercise interventions that yielded increased muscle strength and lean body mass in men with PC on ADT [31, 32].
Based on these results, it is possible that recommending exercise regiments to men undergoing ADT could help prevent loss of muscle mass. Although speculative, this could translate to better overall health and PC outcomes; however, further research is needed. While no widespread evidence-based prevention or treatment strategies currently exist for ADT-associated changes in body composition, these exercise interventions are promising, and could improve not only QOL, but also long-term PC outcomes for men undergoing ADT as noted in a recent guideline to prevent such effects [32].
In addition to exercise, dietary studies might also assist in increasing muscle strength and lean mass. Whey protein has shown to promote gains in lean mass in healthy participants [33] when combined with resistance training, but no studies have looked at the effects of this supplement on men with PC undergoing ADT. In addition, supplements combined with diets modified in macronutrient composition might be effective in reducing ADT-induced adverse effects. For example, in a dietary intervention study, we found men consuming low carbohydrate diets (20 g per day), lost 10.6 kg while completely blocking ADT-induced insulin resistance [34].
Despite the strengths afforded by our study, including a Veterans Affairs cohort with data from multiple centers, there are limitations. First, our study is a retrospective cohort, relying on information extracted from patients’ medical charts. Quality of care and completeness of documented data may have varied during the extended study period resulting in exclusions of potentially eligible patients, particularly those in the earlier time period of the study. Such exclusions raise concerns that selection bias may have been introduced. However, our finding that the distribution of clinical factors, which differed between excluded and included men, was balanced across study weight status groups does not point to compromised internal validity. Second, although we were limited by the existing data in our estimation of weight change following ADT initiation, we have conducted sensitivity analyses with more rigorous time-windows for weight change which was shown to have negligible impact on our results. Third, as detailed body composition measurements were unavailable, we were unable to directly test the hypothesis that patients losing weight were losing muscle mass. Fourth, we did not assess dietary intake or physical activity to determine their impact on our results. Finally, larger studies with longer follow-up, and potentially the testing of longer lag times are needed to confirm our findings.
Conclusion
Among men with PC undergoing ADT at five Veterans Affairs medical centers, weight loss was associated with increased risk of metastases, ACM, and PCSM. Risks associated with moderate-to-severe weight gain and PC progression and PCSM were elevated but not statistically significant. We speculate that increased weight loss post-ADT could be associated predominantly with reduced lean muscle mass; however, validation of these findings and further study of the mechanisms linking weight loss, body composition, and PC outcomes are required.
References
Foulkes SJ, Daly RM, Fraser SF (2017) The clinical importance of quantifying body fat distribution during androgen deprivation therapy for prostate cancer. Endocr Relat Cancer 24:R35–R48
Galvao DA, Spry NA, Taaffe DR, Newton RU, Stanley J, Shannon T et al (2008) Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int 102:44–47
Nguyen PL, Alibhai SM, Basaria S, D’Amico AV, Kantoff PW, Keating NL et al (2015) Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 67:825–836
Hart NH, Galvão DA, Newton RU (2017) Exercise medicine for advanced prostate cancer. Cur Opin Support Palliat Care 11:247–257
Galvao DA, Taaffe DR, Spry N, Joseph D, Turner D, Newton RU (2008) Reduced muscle strength and functional performance in men with prostate cancer undergoing androgen suppression: a comprehensive cross-sectional investigation. Prostate Cancer Prostatic Dis 12:198–203
Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, Schoenfeld DA et al (2002) Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 87:599–603
Tzortzis VSM, Zachos I, Oeconomou A, Pisters L, Bargiota A (2017) Adverse effects of androgen deprivation therapy in patients with prostate cancer: focus on metabolic complications. Hormones 16:115–123
Keating NL, O’Malley AJ, Freedland SJ, Smith MR (2010) Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst 102:39–46
Alibhai SMH, Duong-Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM et al (2009) Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clinl Oncol 27:3452–3458
Kim HS, Moreira DM, Smith MR, Presti JC Jr, Aronson WJ, Terris MK et al (2011) A natural history of weight change in men with prostate cancer on androgen-deprivation therapy (ADT): results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. BJU Int 107:924–928
Braunstein LZ, Chen M-H, Loffredo M, Kantoff PW, D’Amico AV (2014) Obesity and the odds of weight gain following androgen deprivation therapy for prostate cancer. Prostate Cancer 2014:230812
Keto CJ, Aronson WJ, Terris MK, Presti JC, Kane CJ, Amling CL et al (2012) Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: results from the SEARCH database. BJU Int 110:492–498
Freedland SJ, Vidal AC, Howard LE, Terris MK, Cooperberg MR, Amling CL et al (2017) Race and risk of metastases and survival after radical prostatectomy: results from the SEARCH database. Cancer 123:4199–4206
Vidal AC, Howard LE, Sun SX, Cooperberg MR, Kane CJ, Aronson WJ et al (2017) Obesity and prostate cancer-specific mortality after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Prostate Cancer Prostatic Dis 20:72–78
Rodriguez C, Freedland SJ, Deka A, Jacobs EJ, McCullough ML, Patel AV et al (2007) Body mass index, weight change, and risk of prostate cancer in the cancer prevention study II nutrition cohort. Cancer Epidemiol Biomark Prev 16:63–69
Greenland S, Daniel R, Pearce N (2016) Outcome modelling strategies in epidemiology: traditional methods and basic alternatives. Int J Epidemiol 45:565–575
Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C et al (2016) A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol 69:428–435
Allott EH, Masko EM, Freedland SJ (2013) Obesity and prostate cancer: weighing the evidence. Eur Urol 63:800–809
Cao Y, Ma J (2011) Body mass index, prostate cancer–specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Philadelphia) 4:486–501
Guerrios-Rivera L, Howard L, Frank J, De Hoedt A, Beverly D, Grant DJ et al (2017) Is body mass index the best adiposity measure for prostate cancer risk? Results from a Veterans Affairs biopsy cohort. Urology 105:129–135
Isbarn H, Boccon-Gibod L, Carroll PR, Montorsi F, Schulman C, Smith MR et al. Androgen deprivation therapy for the treatment of prostate cancer: consider both benefits and risks. Eur Urol 55:62–75
Seible DM, Gu X, Hyatt AS, Beard CJ, Choueiri TK, Efstathiou JA et al (2014) Weight gain on androgen deprivation therapy: which patients are at highest risk? Urology 83:1316–1321
Buttigliero C, Vana F, Bertaglia V, Vignani F, Fiori C, Osella G et al (2015) The fat body mass increase after adjuvant androgen deprivation therapy is predictive of prostate cancer outcome. Endocrine 50:223–230
Dickerman BA, Ahearn TU, Giovannucci E, Stampfer MJ, Nguyen PL, Mucci LA et al (2017) Weight change, obesity and risk of prostate cancer progression among men with clinically localized prostate cancer. Int J Cancer 141:933–944
Cheung AS, Hoermann R, Dupuis P, Joon DL, Zajac JD, Grossmann M (2016) Relationships between insulin resistance and frailty with body composition and testosterone in men undergoing androgen deprivation therapy for prostate cancer. Euro J Endocrinol 175:229–237
Kiwata JL, Dorff TB, Schroeder ET, Gross ME, Dieli-Conwright CM (2016) A review of clinical effects associated with metabolic syndrome and exercise in prostate cancer patients. Prostate Cancer Prostatic Dis 19:323–332
de Rooy C, Grossmann M, Zajac JD, Cheung AS (2016) Targeting muscle signaling pathways to minimize adverse effects of androgen deprivation. Endocr Relat Cancer 23:R15–R26
Warburton DER, Gledhill N, Quinney A (2001) The effects of changes in musculoskeletal fitness on health. Can J Appl Physiol 26:161–216
Alibhai SMH, Breunis H, Timilshina N, Naglie G, Tannock I, Krahn M et al (2015) Long-term impact of androgen-deprivation therapy on physical function and quality of life. Cancer 121:2350–2357
Bourke L, Doll H, Crank H, Daley A, Rosario D, Saxton JM (2011) Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: a feasibility study. Cancer Epidemiol Biomark Prev 20:647–657
Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG et al (2003) Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 21:1653–1659
Owen PJ, Daly RM, Livingston PM, Fraser SF (2017) Lifestyle guidelines for managing adverse effects on bone health and body composition in men treated with androgen deprivation therapy for prostate cancer: an update. Prostate Cancer Prostatic Dis 20:137–145
Volek JS, Volk BM, Gomez AL, Kunces LJ, Kupchak BR, Freidenreich DJ et al (2013) Whey protein supplementation during resistance training augments lean body mass. J Am Coll Nutr 32:122–135
Freedland S, Aronson W, Howard L, Smith J, Smith M, Stout J et al (2016) A prospective randomized trial of dietary carbohydrate restriction for men initiating androgen deprivation therapy: carbohydrate and prostate study I (CAPS1). J Urol 195:e29–e30
Funding
This study was supported by NIH/NCI Grant Nos. P50CA92131 and NIH K24 CA160653.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Griffin, K., Csizmadi, I., Howard, L.E. et al. First-year weight loss with androgen-deprivation therapy increases risks of prostate cancer progression and prostate cancer-specific mortality: results from SEARCH. Cancer Causes Control 30, 259–269 (2019). https://doi.org/10.1007/s10552-019-1133-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-019-1133-5