Abstract
Purpose
Acute lymphoblastic leukemia (ALL) is a rare hematological malignancy. With the recent introduction of a classification system for hematopoietic and lymphoid neoplasms, more comprehensive assessment of ALL epidemiology is now possible. In this study, we describe recent international incidence of ALL and project the annual number of diagnoses to 2025. We also estimate relative survival and average potential years of life lost (AYLL) to assess the societal burden of ALL.
Methods
Age-specific incidence data for ALL from select cancer registries in different geographies were obtained from the International Agency for Research on Cancer’s Cancer Incidence in Five Continents Database. Country-specific age-standardized rates were calculated to allow for direct comparisons between countries. ALL-specific mortality and relative survival data were only available from the United States (US) National Cancer Institute’s Surveillance, Epidemiology, and End Results program; mortality rates were estimated for other countries.
Results
The age-standardized incidence rate of ALL during 2003–2007 ranged from 1.08 to 2.12 per 100,000 person-years in selected countries. Incidence was generally higher in the Americas and Oceania and lower in Asia and Eastern Europe. In most countries, the incidence rate of ALL in children was approximately four times that in adults. Survival was particularly poor among adults. In selected countries, the estimated AYLL ranged from 30 to 48 years for all ages and from 23 to 39 years for adults.
Conclusions
Although a rare disease, ALL presents a significant public health burden given poor survival outcomes among adults, AYLL, and its importance as the most common pediatric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lymphoblastic leukemia (ALL) is a rare hematological malignancy of the bone marrow in which precursor lymphoblasts, blocked at an early stage of differentiation, proliferate rapidly and supplant normal hematopoietic cells of the bone marrow. The incidence of ALL peaks sharply among children 1–4 years of age and gradually rises again among adults starting around age 50 [1]. ALL comprises <1 % of adult cancers, but represents the most common childhood malignancy, accounting for approximately 25 % of cancers and 80 % of all leukemias in children [1, 2]. The prognosis of ALL can be particularly poor, especially among adults. Although the cure rate in childhood ALL nears 90 % [3, 4], the same level of success has not been achieved in adult ALL. Despite complete remission rates exceeding 90 % with initial chemotherapy treatment [5, 6], the cure rate in adult ALL is only 20–40 % [5, 7, 8]; most adults with ALL eventually experience relapse, often within 1 year of their diagnosis, from which the median survival is only 4–8 months [9–11].
In 2001, the World Health Organization (WHO) produced a consensus-based classification system that defines hematopoietic and lymphoid neoplasms based on distinct combinations of morphology, immunophenotype, genetic, molecular, and clinical features, thereby providing a framework for consistent identification of such malignancies internationally [12]. However, subtypes of leukemia, including ALL, are seldom distinguished in contemporary studies of global cancer incidence, mortality, and survival; instead, global epidemiologic measures of hematologic malignancies are often limited to broader categorizations of cancer, such as “leukemia” [13–15]. Furthermore, studies providing statistics on ALL are typically limited to children, do not differentiate between childhood and adult ALL, are based on older data, or are restricted to a single country or global region [2, 16–29].
The objectives of this study are to provide a comprehensive assessment of recent international incidence of childhood and adult ALL through 2007 and to estimate the number of future childhood and adult ALL diagnoses from 2015 to 2025. In addition, we approximate the incidence of major subtypes of adult ALL, describe current estimates of relative survival in childhood and adult ALL, and provide international estimates of average potential years of life lost (AYLL) due to ALL to assess the societal burden of this grievous illness.
Methods
Incidence data for ALL from select cancer registries in Asia, the Americas, Europe, and Oceania (Table 1) were obtained from three recent volumes of the International Agency for Research on Cancer’s (IARC’s) Cancer Incidence in Five Continents Volume X (CI5-X) database that covers the 5-year time period 2003–2007 [30]. For each registry, incident cases of ALL, categorized according to International Classification of Diseases, 10th Revision (ICD-10) code C91.0, and person-years were abstracted by age group (i.e., 5-year age groups 0–4, 5–9, and 10–14 years, and so on through 80–84 years, as well as a single group for persons 85 years and older). For countries without a national cancer registry, we aggregated data from regional registries to provide a proxy of national incidence. Crude age group-specific, childhood (0–19 years), adult (20–85+ years), and overall incidence rates were calculated for each registry and country. Data from CI5-X were used to provide estimates of global incidence rates of ALL in 21 selected countries across the four global regions. To enable making valid comparisons across countries with different age distributions, crude incidence rates were age-standardized to the World Health Organization’s (WHO’s) 2000–2025 world standard population [31].
In order to estimate the number of expected incident diagnoses of ALL in future years, we utilized country-specific population projections for 2015 through 2025, from The Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat, World Population Prospects: The 2012 Revision [32]. Population figures were gathered by age group as described above. Since identification and reporting of ALL in cancer registries may have changed after the WHO developed its taxonomy of hematologic malignancies in 2001, measures of ALL incidence preceding the establishment of this classification system may not be comparable with measures that followed, and combining earlier (i.e., prior to 2001) with more recent data may misinform historical trends of ALL incidence. Therefore, in order to conservatively project annual ALL incidence in the coming years, we assumed that future age-specific incidence rates for each country would be the same as those reported in CI5-X. Thus, to project incident diagnoses of ALL for years 2015, 2020, and 2025, crude age group-specific incidence rates of ALL from CI5-X were multiplied by the population projections for each country; for countries without a national cancer registry, incidence rates from the aggregation of regional registry data were used. Within each calendar year, incidence estimates were summed across age groups to provide the number of projected diagnoses overall and separately for children and adults.
Incidence estimates of adult ALL for 2015 were further divided into major subtypes of ALL according to immunophenotype and Philadelphia chromosome status. Since information on immunophenotype and Philadelphia chromosome status is typically absent from cancer registry data, literature-based approximations of these disease characteristics were applied to our incidence estimates for 2015. Estimates were first divided according to immunophenotype, with approximately 80 % B-lineage and the remaining 20 % T-lineage [33–37]; B-lineage was further divided into Burkitt leukemia (5 % of adult ALL), which is treated differently from other forms of ALL, and precursor B cell ALL (75 % of adult ALL). Precursor B cell ALL estimates were then divided according to Philadelphia chromosome status, with approximately 23 % Philadelphia chromosome positive (Ph+) and the remaining 77 % Philadelphia chromosome negative (Ph−) [38]. To further describe the largest subgroup of adult ALL patients with a significant unmet medical need, we estimated the number of adults with incident Ph− precursor B cell ALL who will experience refractory or relapsed disease by applying proportions from published trial results to our incidence estimates [9, 39, 40].
Survival data from 18 cancer registries participating in the United States (US) National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program [41] were used to estimate 1- and 5-year relative survival. Survival data were limited to the US as the SEER program database is currently the only population-based resource that provides survival data for ALL specific to 5-year age groups. To utilize the most recent information available in the SEER program, we obtained relative survival data for patients with a diagnosis of ALL in the SEER 18 database between 2003 and 2010 and followed through 2011. We excluded patients with ALL reported by death certificate or autopsy only and patients with unknown survival time. One- and 5-year relative survival estimates were provided for each age group, as well as for childhood, adult, and overall populations.
To estimate the projected AYLL from ALL in 2015, we obtained ALL-specific mortality rates for the 18 SEER cancer registries during the period 2003–2007 from the SEER mortality database [42]. ALL incidence rates were estimated from the SEER database for the corresponding 18 cancer registries and time period, and ratios of incidence to mortality rates for each 5-year age group were calculated. In order to approximate mortality rates for other countries, these ratios of incidence to mortality rates in the USA were assumed to be similar to those in other countries. Mortality rates were approximated by dividing age-specific incidence to mortality rate ratios by the corresponding age-specific incidence rates from CI5-X for the selected countries. These approximated country-specific mortality rates were multiplied by population projections to estimate the number of expected deaths from ALL in 2015. After extracting estimated life expectancies for each country from the Global Health Observatory data repository of the WHO [43], potential years of life lost (PYLL) were calculated by multiplying the number of estimated ALL-specific deaths for a given 5-year age group by the remaining life expectancy for that group [44]. For each country, the total PYLL for children, adults, and the overall population were approximated by summing the PYLL across appropriate age groups. The AYLL from ALL for children, adults, and the overall population were then estimated as the total PYLL divided by the corresponding total number of estimated ALL deaths.
To evaluate whether our assumption of using US-based ratios of incidence to mortality rates provides fair approximations of mortality rates for other countries, particularly for the purpose of estimating the AYLL from ALL, we compared our estimated mortality rates with those for countries in which we were able to identify country-specific mortality data [45–49]. Specifically, we compared mortality rates for each 5-year age group, the distribution of mortality across age groups, and estimates of the AYLL from ALL.
Results
Age-specific incidence of ALL
Figure 1 shows incidence rates of ALL for 5-year age groups for a few countries (Australia, China, Costa Rica, Germany, Italy, Japan, and the USA) selected to represent the various geographic regions; age-specific incidence curves for the remaining countries included in this study followed the same pattern observed in the figure (results not shown). Incidence of ALL peaked in children aged 0–4 years and then decreased sharply among older children and adolescents before leveling off in young adults. Incidence was generally lowest between adult age groups 25–29 and 45–49 years, and increased gradually among older age groups starting among adults 50–54 years.
Overall incidence of ALL
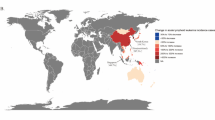
In CI5-X, an approximately twofold difference in overall ALL age-standardized incidence was observed between Costa Rica (2.12 per 100,000 person-years) and the Czech Republic (1.08 per 100,000), the countries with the highest and lowest rates, respectively, among those we selected for our analyses (Fig. 2a). Generally, the age-standardized incidence of ALL was highest in the Americas and Oceania and lowest in Asia (except Singapore) and Eastern Europe (i.e., Czech Republic and Poland).
Incidence of ALL in children (0–19 years)
Among selected countries, Singapore reported the highest age-standardized incidence rate of childhood ALL (3.78 per 100,000 person-years) (Fig. 2b). Age-standardized incidence rates of >3.5 per 100,000 were also reported in Australia, Costa Rica, and Italy. Conversely, China reported the lowest age-standardized incidence rate of childhood ALL (2.17 per 100,000), while other Asian countries, the Czech Republic, and Poland also reported age-standardized rates of <2.4 per 100,000.
Incidence of ALL in adults (20+ years)
On average, the incidence rate of adult ALL among selected countries was roughly four times lower than the corresponding incidence rate of childhood ALL (Fig. 2c). The age-standardized incidence of adult ALL was remarkably higher in Colombia and Costa Rica than in all other selected countries. There was greater than a threefold difference in the age-standardized incidence of adult ALL between Colombia (1.47 per 100,000) and the Czech Republic (0.43 per 100,000), the countries with the highest and lowest reported rates, respectively.
Projected incident diagnoses of ALL
Under the assumption that future age-specific incidence rates of ALL will be similar to those estimated in CI5-X for the 2003–2007 period, the projected annual number of incident diagnoses of ALL will generally increase between 2015 and 2025 in tandem with anticipated population increases over this time period (Table 2). Overall, proportional growth in the estimated number of incident diagnoses of ALL is highest for Australia, Colombia, and Singapore and lowest for Japan, where the total number of ALL diagnoses is projected to decrease over time. The number of incident diagnoses of childhood ALL is only expected to increase in a few countries (i.e., France, Sweden, the UK, Singapore, Australia, and the USA), whereas the number of incident diagnoses of adult ALL is projected to grow in each of the selected countries other than the Czech Republic, Denmark, Poland, and New Zealand.
Projected incidence of selected adult ALL subtypes
The projected incidence of adult ALL in 2015 for the USA and European Union Five (EU5), overall and by selected subtypes, is shown in Fig. 3. Between 2150 and 2370 and 1920–2120 incident diagnoses of adult ALL were projected for the USA and EU5, respectively. Of these, approximately 1720–1900 in the USA and 1540–1700 in the EU5 were estimated as B-lineage, the primary immunophenotype, including 1610–1780 in the USA and 1440–1600 in the EU5 as precursor B cell ALL specifically. Among the projections for precursor B cell ALL, the majority were estimated as Ph− (1240–1370 in the USA and 1120–1230 in the EU5), and of those, an estimated 50 % (620–685 in the USA and 560–615 in the EU5) will be refractory to initial chemotherapy or eventually experience relapse.
Relative survival
Among patients diagnosed with ALL in one of the 18 SEER regions of the USA between 2003 and 2010 and followed through 2011, the overall 1- and 5-year relative survival rates were 82.7 and 66.3 %, respectively (Fig. 4). Infants (<1 year) had less favorable survival than older children (1–4, 5–9, and 10–14 years) and adolescents (15–19 years). This aside, relative survival was higher among children (0–19 years) than in adults (20+ years). Beginning at ages 1–4 years, relative survival decreased progressively with increasing age at ALL diagnosis, with a particularly notable decline in 5-year survival beginning at ages 20–24 years. Five-year relative survival was <50 % in adults 20–24 years at diagnosis and declined to <30 % among adults 50–54 years and older. Survival in adults 65 years and older was the poorest with 1- and 5-year relative survival rates of 35 and 12 %, respectively. Relative survival estimates are also provided in Table 3.
Average potential years of life lost
Estimated AYLL due to ALL in 2015 based on approximated mortality rates are displayed in Table 4 for each population (i.e., overall, children, and adults) with countries ordered according to life expectancy from birth. Overall, the estimated AYLL range from a low of 29.8 years in Poland to a high of 48.2 years in Israel with approximately half of the selected countries having estimated AYLL of 35–40 years. Among children, the estimated AYLL vary from 66.5 years in Brazil to 75.8 years in Japan. For most countries, the AYLL in children were estimated to be between 70 and 75 years. Among adults, AYLL range from 22.5 years in Poland to 38.9 years in Israel with approximately half of selected countries having estimated AYLL of 25–30 years.
For countries with available data, mortality rates from country-specific sources and those approximated using SEER-based incidence to mortality rate ratios and incidence rates from CI5-X are provided in Table 5 in “Appendix.” Compared to estimates from country-specific sources for Australia, Denmark, Spain, and the UK, approximated mortality rates were generally higher for most age groups, resulting in more estimated deaths and PYLL. The distribution of the number of deaths across age groups was similar between estimates from country-specific sources and those that we approximated. Among adults, approximated mortality rates estimate 18–23, 33–40, and 40–49 % of ALL deaths occur among those aged 20–39, 40–64, and 65+ years, respectively; in comparison, country-specific sources estimate 16–24, 30–34, and 46–53 % of adult ALL deaths occur among these age groups, respectively. Estimates of the AYLL from ALL were also quite similar between those we approximated and those from country-specific sources. Absolute differences in estimates ranged from 0.1 to 0.8 years for childhood ALL, 0.3–1.8 years for adult ALL, and 1.2–2.4 years for ALL overall.
Discussion
In this study, we provide a comprehensive assessment of the international incidence of childhood and adult ALL using data more recent than previously available and that followed the introduction of the 2001 WHO classification system for hematologic malignancies. In addition, we estimate the number of future childhood and adult ALL diagnoses, including approximations for selected subtypes of adult ALL, describe current estimates of relative survival in childhood and adult ALL, and provide international estimates of the AYLL from ALL to illustrate the public health burden of this grievous illness.
In agreement with previous studies [2, 50], the incidence of ALL varied considerably by age with some variations across geographic regions. In the majority of selected countries, the age-standardized incidence rate of ALL among children was three to four times the incidence rate among adults. However, the variation in international incidence was minimal, considering that the majority of selected countries reported an age-standardized incidence rate between 1.1 and 1.8 per 100,000 person-years; only Colombia and Costa Rica reported an age-standardized incidence rate of >1.8 per 100,000. The elevated overall incidence rates in Colombia and Costa Rica largely reflect the markedly higher frequency of adult ALL in these two countries and are consistent with past reports of elevated incidence among countries in Latin America and Hispanic populations in the USA [2, 20, 28, 51]. Likewise, the lower rates of ALL observed in the Czech Republic and Poland are also in line with findings from earlier studies in which both the overall incidence of ALL [23] and the incidence of childhood ALL [27] were less frequent in Eastern Europe relative to other European regions. Of note, however, a recent study in Croatia [52] reported an age-standardized incidence rate of ALL >1.6 per 100,000 person-years, which was higher than the rate reported for Poland and the Czech Republic in this study.
Quantification of the projected number of annual ALL diagnoses is central to understanding the societal burden of this disease. In particular, estimates of the number of annual diagnoses for subgroups of patients with a significant unmet medical need can inform research, health policy, and healthcare resource allocation priorities. Here, we provided projections of the incidence of overall, childhood, and adult ALL, including estimates for select subtypes of adult ALL. Based on expected population changes, we projected that the number of diagnoses of ALL in the majority of selected countries will remain fairly stable or increase slightly between 2015 and 2025. However, with the aging of populations across the globe, we estimated that the number of diagnoses of childhood ALL will decrease over this time period, particularly in Brazil, China, and Japan where the populations of children are expected to decline. Conversely, with an increase in the number of adults, especially older adults, projected for most countries, we estimated that the number of diagnoses of adult ALL will increase between 2015 and 2025; this projection indicated an increase in adult ALL diagnoses of at least 20 % in Brazil, Colombia, and Singapore in particular, but estimated an increase of <15 % for all other selected countries. Given that recent studies [21, 52, 53] reported trends of stable incidence for ALL in several countries, we believe that our use of incidence rates from CIV to X to project the number of diagnoses of ALL from 2015 to 2025 provides conservative estimates of the incidence of ALL in selected countries. In addition, by using incidence rates specific to 5-year age groups, we allowed the incidence rate of ALL overall and for the broader age groupings (i.e., childhood and adults) to still vary over time based on the projected changes in the age distribution of the populations.
We showed that survival rates in ALL differ markedly by age. In particular, infants and older adults have less favorable survival than other children, adolescents, and younger adults. Differences in survival may be partly explained by variation in other prognostic factors and the treatments utilized across age groups [54]. For example, pediatric patients are typically treated more aggressively than adults, and Philadelphia chromosome-positive ALL, which is more common among adults [55], has traditionally been associated with a particularly poor prognosis. Furthermore, the low survival observed among adults 65 and older may reflect greater comorbidity and frailty in older patients. In turn, greater comorbidity and frailty may decrease the tolerability of treatment-associated toxicities and prohibit the use of standard treatment regimens in older patients. Nevertheless, poor survival among adults with ALL, including both younger and older adults (5-year relative survival was <50 % even among adults 20–24 years of age) further illustrates the burden of this disease and highlights the unmet medical need to develop better treatments for this patient population.
Although ALL is a rare form of cancer, the peak in incidence among children and poor long-term survival among adults contributes to a comparatively high AYLL that exceeds those of other more common malignancies. In the UK, for example, we estimated that adults who die with ALL lose 28 years of life on average, higher than the AYLL reported for other malignancies, including non-Hodgkin lymphoma (13 years) and prostate (6 years), lung (12 years), and breast (14 years) cancers [44]. Similarly, in selected countries, the AYLL estimated for adults with ALL are greater than the AYLL reported for adults with myeloma (17 years) in North America, Japan, and Europe [56]. The higher AYLL in ALL relative to other cancers among adults illustrate that adult ALL is largely a disease of younger, working age adults; in the SEER mortality database [42] from 2003 to 2007, more than 40 % of adult ALL deaths occurred among adults <50 years of age and approximately 60 % of adult ALL deaths occurred in adults under age 65 (results not shown). Thus, although the annual incidence rate, number of deaths, and the total PYLL for ALL are small relative to other cancer types, the burden of ALL is remarkably high in comparison when we consider the poor survival outcomes in adults, the relatively young age at diagnosis, and the AYLL among patients who die of this grievous disease.
It should be noted that our estimates of AYLL from ALL varied across countries, particularly among adults. However, the variation in AYLL across countries is likely due in large part to differences in life expectancy and the age distribution of the population, rather than differences in age-specific mortality due to ALL. For example, the difference in life expectancy at birth between Japan and Brazil (10 years) is similar to the difference in AYLL among children who die from ALL in these countries (~9 years); in general, as life expectancy at birth decreases, so does the estimated AYLL among children. Similarly, estimates of AYLL from ALL among adults are generally higher in countries where adults 65 and older make up smaller proportions of the adult population. For example, adults 65 and older make up <20 % of the adult population and 20 % or less of the incidence of adult ALL in Israel, Singapore, and Costa Rica, the countries with the three highest estimates for AYLL among adults; conversely, adults 65 and older make up over 25 % of the adult population and approximately 45 % or more of the incidence of adult ALL in Germany, Italy, and Japan, countries with three of the lowest estimates for AYLL. Thus, despite similar age-specific incidence rate patterns, the age distribution of adult ALL, and consequently the estimates of AYLL, can vary slightly across countries.
Utilization of the most recent data available from CI5 to estimate the international incidence of ALL is a notable strength of this analysis as is the provision of separate estimates of overall, childhood, and adult ALL incidence. In addition, this study projects the annual incidence of ALL in future years and is, to our knowledge, the first to estimate the average number of potential years of life lost to ALL, a valuable measure of the burden of this disease. Still, the conclusions drawn in the study are somewhat constrained by the limitations in the data available. For instance, given the lack of national cancer registries in many countries, the CI5 database is largely comprised of data from regional cancer registries that may only cover small subnational areas; it is possible that measures of cancer incidence in the regional registries included in CI5 do not always accurately reflect patterns of incidence in the entire country. Furthermore, given that ALL is a rare malignancy, the trends reported in regional registries with particularly low incidence rates warrant cautious interpretation as they are based on small numbers of diagnoses and are susceptible to random variation. The lack of mortality and survival data for countries other than the USA is another important limitation of this study. As such, to estimate mortality rates and AYLL for other countries, we relied on US age-specific ratios of incidence to mortality rates, which essentially assumes similar survival rates across countries. Thus, if the true survival rates in other countries differ greatly from those observed in the USA, we may have underestimated or overestimated mortality rates for some age groups and in turn underestimated or overestimated country-specific deaths and years of life lost from ALL. However, when we compared our estimated mortality rates with those from country-specific sources, we found little difference in regard to the age distribution of mortality or the estimation of AYLL. In addition, it is important to note that although accurate mortality rates are necessary to estimate the actual number of deaths and the total years of life lost, the estimation of AYLL is not dependent on the accuracy of these measures, but rather the distribution of the number of estimated deaths across age groups. Further, our estimates of 5-year relative survival among children and adults with ALL in the USA are similar to those from recent published survival estimates for ALL in Japan and European countries [25, 34, 57, 58]. Therefore, given similar patterns of incidence and survival across age groups for ALL, we believe that ratios of incidence to mortality rates from a single country such as the USA provide reasonable approximations of mortality rates in other countries solely for the purpose of estimating AYLL.
In summary, there is variation in the incidence of ALL by age and geographic region. However, age-standardized incidence estimates of ALL in our study only ranged from 1 to 2 per 100,000 people, whereas previous research [29] estimated a wider range of 1–4.75 per 100,000, perhaps suggesting that more recent data reflect greater consistency in the identification and reporting of ALL across nations since the WHO development of a consensus-based classification system for hematologic malignancies. In addition, despite long-term survival rates near 90 % among children, survival remains particularly poor in adults. The noted effect of older age on survival and the substantial AYLL among adults with ALL informs on the burden of this grievous disease and highlights the need to develop better treatments.
References
Wartenberg D, Groves FD, Adelman AS (2008) Acute lymphoblastic leukemia: epidemiology and etiology. In: Estey EH, Faderl S, Kantarjian H (eds) Acute leukemias, 1st edn. Springer, Berlin, pp 77–93
Groves FD, Linet MS, Devesa SS (1995) Patterns of occurrence of the leukaemias. Eur J Cancer 31a:941–949
Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ et al (2012) Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol 30:1663–1669. doi:10.1200/jco.2011.37.8018
Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC et al (2009) Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 360:2730–2741. doi:10.1056/NEJMoa0900386
Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM et al (2005) Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood 106:3760–3767. doi:10.1182/blood-2005-04-1623
Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E et al (2009) Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol 27:911–918. doi:10.1200/jco.2008.18.6916
Sive JI, Buck G, Fielding A, Lazarus HM, Litzow MR, Luger S et al (2012) Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. Br J Haematol 157:463–471. doi:10.1111/j.1365-2141.2012.09095.x
Thomas DA, O’Brien S, Faderl S, Garcia-Manero G, Ferrajoli A, Wierda W et al (2010) Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol 28:3880–3889. doi:10.1200/jco.2009.26.9456
Oriol A, Vives S, Hernandez-Rivas JM, Tormo M, Heras I, Rivas C et al (2010) Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica 95:589–596. doi:10.3324/haematol.2009.014274
Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G et al (2007) Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 109:944–950. doi:10.1182/blood-2006-05-018192
Gökbuget N, Stanze D, Beck J, Diedrich H, Horst HA, Huttmann A et al (2012) Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 120:2032–2041. doi:10.1182/blood-2011-12-399287
Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES (2011) The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 117:5019–5032. doi:10.1182/blood-2011-01-293050
American Cancer Society (2011) Global cancer facts and figures, 2nd edn. American Cancer Society, Atlanta
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917. doi:10.1002/ijc.25516
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90. doi:10.3322/caac.20107
Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM (2012) Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood 119:34–43. doi:10.1182/blood-2011-04-347872
Dalmasso P, Pastore G, Zuccolo L, Maule MM, Pearce N, Merletti F et al (2005) Temporal trends in the incidence of childhood leukemia, lymphomas and solid tumors in north-west Italy, 1967–2001. A report of the Childhood Cancer Registry of Piedmont. Haematologica 90:1197–1204
Linabery AM, Ross JA (2008) Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer 112:416–432. doi:10.1002/cncr.23169
Parkin DM, Stiller CA, Draper GJ, Bieber CA (1988) The international incidence of childhood cancer. Int J Cancer 42:511–520
Yamamoto JF, Goodman MT (2008) Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997–2002. Cancer Causes Control 19:379–390. doi:10.1007/s10552-007-9097-2
Chihara D, Ito H, Matsuda T, Shibata A, Katsumi A, Nakamura S et al (2014) Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol 164:536–545. doi:10.1111/bjh.12659
Marcos-Gragera R, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Maynadie M et al (2011) Survival of European patients diagnosed with lymphoid neoplasms in 2000–2002: results of the HAEMACARE project. Haematologica 96:720–728. doi:10.3324/haematol.2010.034264
Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O et al (2010) Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood 116:3724–3734. doi:10.1182/blood-2010-05-282632
De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D et al (2014) Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5—a population-based study. Lancet Oncol 15:23–34. doi:10.1016/s1470-2045(13)70546-1
Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel J et al (2014) Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5—a population-based study. Lancet Oncol 15:35–47. doi:10.1016/s1470-2045(13)70548-5
Svendsen AL, Feychting M, Klaeboe L, Langmark F, Schuz J (2007) Time trends in the incidence of acute lymphoblastic leukemia among children 1976–2002: a population-based Nordic study. J Pediatr 151:548–550. doi:10.1016/j.jpeds.2007.07.006
Coebergh JW, Reedijk AM, de Vries E, Martos C, Jakab Z, Steliarova-Foucher E et al (2006) Leukaemia incidence and survival in children and adolescents in Europe during 1978–1997. Report from the Automated Childhood Cancer Information System project. Eur J Cancer 42:2019–2036. doi:10.1016/j.ejca.2006.06.005
Fajardo-Gutierrez A, Juarez-Ocana S, Gonzalez-Miranda G, Palma-Padilla V, Carreon-Cruz R, Ortega-Alvarez MC et al (2007) Incidence of cancer in children residing in ten jurisdictions of the Mexican Republic: importance of the Cancer registry (a population-based study). BMC Cancer 7:68. doi:10.1186/1471-2407-7-68
Redaelli A, Laskin BL, Stephens JM, Botteman MF, Pashos CL (2005) A systematic literature review of the clinical and epidemiological burden of acute lymphoblastic leukaemia (ALL). Eur J Cancer Care (Engl) 14:53–62. doi:10.1111/j.1365-2354.2005.00513.x
Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kholer B, Piñeros M et al (2013) Cancer Incidence in Five Continents Volume X (IARC Scientific Publications No. 164). Lyon, IARC
Ahmad OB, Boschi-Pinto C, López AD, Murray CJLLR, Inoue M (2001) Age standardization of rates: a new WHO standard. World Health Organization, Geneva
United Nations, Department of Economic and Social Affairs, Population Division (2013) World Population Prospects: The 2012 Revision. http://esa.un.org/wpp/unpp/panel_indicators.htm. Accessed Feb 21, 2014
Chiaretti S, Vitale A, Cazzaniga G, Orlando SM, Silvestri D, Fazi P et al (2013) Clinico-biological features of 5202 patients with acute lymphoblastic leukemia enrolled in the Italian AIEOP and GIMEMA protocols and stratified in age cohorts. Haematologica 98:1702–1710. doi:10.3324/haematol.2012.080432
Toft N, Schmiegelow K, Klausen TW, Birgens H (2012) Adult acute lymphoblastic leukaemia in Denmark. A national population-based retrospective study on acute lymphoblastic leukaemia in Denmark 1998–2008. Br J Haematol 157:97–104. doi:10.1111/j.1365-2141.2011.09020.x
Li X, Li J, Hu Y, Xie W, Du W, Liu W et al (2012) A comprehensive cytogenetic classification of 1466 Chinese patients with de novo acute lymphoblastic leukemia. Leuk Res 36:720–726. doi:10.1016/j.leukres.2011.12.016
Chen B, Wang YY, Shen Y, Zhang WN, He HY, Zhu YM et al (2012) Newly diagnosed acute lymphoblastic leukemia in China (I): abnormal genetic patterns in 1346 childhood and adult cases and their comparison with the reports from Western countries. Leukemia 26:1608–1616. doi:10.1038/leu.2012.26
Dugas M, Messerer D, Hasford J, Haferlach T, Heinze B, Ludwig W et al (2003) German multicenter study group for adult ALL (GMALL): recruitment in comparison to ALL incidence and its impact on study results. Ann Hematol 82:83–87. doi:10.1007/s00277-002-0585-x
Moorman AV, Harrison CJ, Buck GA, Richards SM, Secker-Walker LM, Martineau M et al (2007) Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood 109:3189–3197. doi:10.1182/blood-2006-10-051912
Tavernier E, Boiron JM, Huguet F, Bradstock K, Vey N, Kovacsovics T et al (2007) Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia 21:1907–1914. doi:10.1038/sj.leu.2404824
Kantarjian H, Thomas D, O’Brien S, Cortes J, Giles F, Jeha S et al (2004) Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer 101:2788–2801. doi:10.1002/cncr.20668
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 18 Regs Research Data + Hurrican Katrina Impacted louisiana Cases, Nov 2013 Sub (1973–2011 varying)—Linked to County Attributes—Total U.S., 1969–2012 Counties, National Cancer Instisute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014 (updated 5/7/2017), based on the November 2013 submission
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality—All COD, Aggregated With State, Total U.S. (1969–2010) <Katrina/Rita Population Adjustment>, National Cancer Instisute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013. Underlying mortality data provided by NCHS (www.cdc.gov/nchs)
World Health Organization (2013) Global Health Observatory Data Repository. http://apps.who.int/ghodata/. Accessed May 19, 2014
Burnet NG, Jefferies SJ, Benson RJ, Hunt DP, Treasure FP (2005) Years of life lost (YLL) from cancer is an important measure of population burden—and should be considered when allocating research funds. Br J Cancer 92:241–245. doi:10.1038/sj.bjc.6602321
Office for National Statistics (2015) Causes of Death. http://www.ons.gov.uk/ons/taxonomy/index.html?nscl=Causes+of+Death#tab-data-tables. Accessed Jan 8, 2015
NHS National Services Scotland, Information Services Division (2015) Data tables: cancer mortality by year. http://www.isdscotland.org/Health-Topics/Cancer/Publications/data-tables.asp?id=1314#1314. Accessed Jan 8, 2015
Instituto Nacional de Estadística (2015) Health: Deaths statistic according to cause of death. http://www.ine.es/jaxi/menu.do?type=pcaxis&path=%2Ft15%2Fp417&file=inebase&L=1. Accessed Jan 8, 2015
Australian Institute of Health and Welfare (AIHW) (2014) Australian Cancer Incidence and Mortality (ACIM) books: acute lymphoblastic leukemia. AIHW, Canberra. http://www.aihw.gov.au/acim-books. Accessed Jan 8, 2015
Ehngholm G, Ferlay J, Christensen N, Kejs AMT, Johannesen TB, Khan S, et al (2014) NORDCAN: Cancer incidence, mortality, prevalence and survival in the Nordic countries, version 7.0. Association of the Nordic Cancer Registries. Danish Cancer Society. http://www.ancr.nu. Accessed Jan 8, 2015
Linet MS, Dores GM, Kim CJ, Devesa SS, Morton LM (2013) Epidemiology and hereditary aspects of acute leukemia. In: Wiernik PH, Goldman JM, Dutcher J (eds) Neoplastic diseases of the blood, 5th edn. Springer, New York, pp 199–212
Perez-Saldivar ML, Fajardo-Gutierrez A, Bernaldez-Rios R, Martinez-Avalos A, Medina-Sanson A, Espinosa-Hernandez L et al (2011) Childhood acute leukemias are frequent in Mexico City: descriptive epidemiology. BMC Cancer 11:355. doi:10.1186/1471-2407-11-355
Novak I, Jaksic O, Kulis T, Batinjan K, Znaor A (2012) Incidence and mortality trends of leukemia and lymphoma in Croatia, 1988–2009. Croat Med J 53:115–123
Hjalgrim LL, Rostgaard K, Schmiegelow K, Soderhall S, Kolmannskog S, Vettenranta K et al (2003) Age- and sex-specific incidence of childhood leukemia by immunophenotype in the Nordic countries. J Natl Cancer Inst 95:1539–1544
Rowe JM (2010) Prognostic factors in adult acute lymphoblastic leukaemia. Br J Haematol 150:389–405. doi:10.1111/j.1365-2141.2010.08246.x
Faderl S, O’Brien S, Pui CH, Stock W, Wetzler M, Hoelzer D et al (2010) Adult acute lymphoblastic leukemia: concepts and strategies. Cancer 116:1165–1176. doi:10.1002/cncr.24862
Ludwig H, Bolejack V, Crowley J, Blade J, Miguel JS, Kyle RA et al (2010) Survival and years of life lost in different age cohorts of patients with multiple myeloma. J Clin Oncol 28:1599–1605. doi:10.1200/jco.2009.25.2114
Sant M, Minicozzi P, Mounier M, Anderson LA, Brenner H, Holleczek B et al (2014) Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. Lancet Oncol 15:931–942. doi:10.1016/s1470-2045(14)70282-7
Horibe K, Saito AM, Takimoto T, Tsuchida M, Manabe A, Shima M et al (2013) Incidence and survival rates of hematological malignancies in Japanese children and adolescents (2006–2010): based on registry data from the Japanese Society of Pediatric Hematology. Int J Hematol 98:74–88. doi:10.1007/s12185-013-1364-2
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The manuscript does not contain clinical studies, patient data, or research involving human participants or animals.
Conflict of interest
A.J.K., V.M.C., and M.A.K. are employees of Amgen Inc. W.M.S. is an employee of Amgen Ltd.
Appendix
Appendix
See Table 5.
Rights and permissions
About this article
Cite this article
Katz, A.J., Chia, V.M., Schoonen, W.M. et al. Acute lymphoblastic leukemia: an assessment of international incidence, survival, and disease burden. Cancer Causes Control 26, 1627–1642 (2015). https://doi.org/10.1007/s10552-015-0657-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-015-0657-6