Abstract
Purpose
Germany lacks an up-to-date assessment of the cancer burden attributable to alcohol. Therefore, cancer incidence attributable to this exposure was estimated for colorectal, liver, breast, and upper aerodigestive tract (UADT) cancer. Additionally, the impact of alcohol on UADT cancer was analyzed by smoking status, to account for synergistic interactions between these two risk factors.
Methods
Alcohol consumption and smoking prevalence from a nationwide survey in Germany 2008–2011 were combined with relative risks of incident cancer from meta-analyses to obtain population attributable risks (PARs), indicating the proportion of cancers that could be avoided by eliminating a risk factor. Each PAR was multiplied with the respective cancer incidence for 2010 to calculate the absolute number of attributable cases.
Results
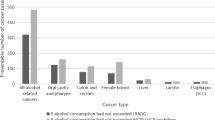
In Germany, for the year 2010, approximately 13,000 incident cancer cases could be attributed to alcohol consumption (3 % of total cases). PAR was highest for esophageal cancer (men: 47.6 % and women: 35.8 %) and lowest for colorectal cancer in men (9.7 %) and breast cancer in women (6.6 %). Among women, moderate consumption levels account for the greatest PAR overall, whereas heavy drinking contributes considerably to overall PAR among men. Additionally, moderate-to-heavy drinking among smokers substantially contributes to the overall PAR of UADT cancers compared to drinking among non-smokers.
Conclusion
In Germany, a substantial proportion of cases of common cancers can be attributed to alcohol consumption, even when consumed at moderate levels. Alcohol consumption with concurrent tobacco smoking is especially important for cancers of the UADT. These findings strengthen the rationale for prevention measures that address exposure at all levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcohol consumption is a major modifiable risk factor for cancers of the upper aerodigestive tract (UADT), as well as for colorectal, liver, and female breast cancers [1]. The carcinogenic properties of alcohol are not fully understood, but consumption can lead to DNA-damage, chronic inflammation, changes in tissues or hormone levels, and increased oxidative stress. Acetaldehyde, which results from the digestion of ethanol, has mutagenic properties and causes DNA-damage, especially in the oropharynx, esophagus, and liver [2, 3].
While alcohol is an independent risk factor for colorectal, liver, and breast cancer [1], an additional synergistic interaction with tobacco for UADT cancers has been observed in epidemiological studies [4–6]. Both alcohol and tobacco can promote carcinogenesis in the UADT in the absence of the other factor, but it has been hypothesized that alcohol additionally acts as a solvent for other carcinogenic agents, like tobacco smoke, and enhances their detrimental effects. This interaction should be considered in calculations of alcohol-attributable disease burden for cancers of the UADT, for example, when calculating population attributable risks (PARs).
Previous analyses of cancer burden attributable to alcohol in Germany did not consider interaction with smoking in UADT cancers [7] or date from the 1990s and focused on cancer-specific mortality [8]. Recent studies, however, have shown that alcohol consumption and smoking prevalence have decreased since then [9, 10]. Thus, the previous estimates of the alcohol-attributable cancer burden in Germany are likely outdated.
This study combines recent data on alcohol and tobacco consumption with relative risk estimates from published meta-analyses to calculate the proportion of incident cancers that could be attributed to alcohol consumption for all alcohol-associated cancers. Additionally, we estimated the exposure level-specific attributable incidence and conducted stratified analyses by smoking status for cancers of the UADT in order to consider the synergistic effect of combined alcohol and tobacco consumption in these cancer types.
Materials and methods
PARs were calculated for the following cancer types (ICD-10 codes in parentheses): oral cavity (C00–C06, C09, and C10), pharynx (C12–C14), squamous cell carcinoma (SCC; ICD-O-3 morphology codes 8050–8084) of the esophagus (C15), larynx (C32), colon-rectum (C18–20), liver (C22), and female breast (C50). These cancer types were selected according to the evaluation of carcinogenic risks to humans by the International Agency for Research on Cancer (IARC) and represent those cancer types for which an association with alcohol or a joint effect of alcohol, and tobacco was deemed convincing [1]. We limited the analyses of the joint effect of alcohol and tobacco to cancers of the UADT since neither the evaluation by the IARC nor other subsequent studies found any interactive effects between alcohol and tobacco for colorectal, liver, and breast cancer [4, 11]. In the following, UADT cancers include oral cavity, pharyngeal, esophageal, and laryngeal cancers.
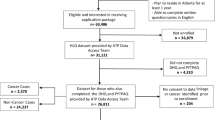
PARs were calculated for men and women ≥35 years of age diagnosed with one of the above mentioned cancers in Germany in the year 2010. To this end, information from three sources was combined: (1) the distribution of alcohol drinking and tobacco smoking from a national, representative survey of adults in Germany, (2) risk estimates for the association between alcohol drinking and the considered cancer types obtained from published meta-analyses, and (3) cancer incidence rates estimated from German cancer registry data. These three components are described in detail in the following sections.
Prevalence of alcohol drinking and tobacco smoking in Germany
Data on the prevalence of alcohol drinking and tobacco smoking among residents in Germany were obtained from the German Health Interview and Examination Survey for Adults (DEGS1). The study methods have been fully reported elsewhere [12]. Briefly, the survey covered a nationwide sample of the adult population in Germany aged 18–79 years and aimed to describe the health status and health behavior of residents. From 2008 to 2011, subjects took part in examinations and/or completed a validated questionnaire about relevant health issues as well as their alcohol and tobacco consumption habits. The prevalence data have been weighted to assure agreement with German national population statistics as of 31 December 2010 regarding sex, age, federal state, German/non-German nationality, community size, and education [12].
Alcohol consumption was assessed with a validated food frequency questionnaire (FFQ) covering the 4-week period prior to the interview [13]. The amount of alcohol consumption was quantified as bottles or glasses of specific beverage types (beer, wine/champagne, liquor, and cocktails/alcoholic mixed drinks). Frequency of consumption was assessed as the frequency per month, week or day with which each specific type of alcoholic beverage was drunk. This information was transformed into average grams of alcohol consumed per day considering the most common percent of alcohol by volume for each type of beverage as well as the alcohol density. Alcohol consumption was then categorized according to levels of intake consistent with the ones used in meta-analyses that contributed relative risk estimates and was stratified by age. In the following, we also discriminate between moderate drinking (<3 drinks per day) and heavy drinking (at least 3 drinks per day). We defined three drinks as containing more than 24 ml or 30 g of alcohol.
Smoking habits were assessed with questions about smoking status and the current or, for ex-smokers, former number of cigarettes smoked per day. For analyzing the impact of alcohol on UADT cancers among smokers and non-smokers, smoking and drinking habits were cross-tabulated according to the categories used in the selected meta-analyses.
Relative risks
Relative risk (RR) estimates concerning the association between either alcohol or alcohol and tobacco and each cancer type were derived from meta-analyses [6, 14–17]. They were identified using the PubMed database with the following MeSH-terms: alcohol drinking/consumption and liver, breast or colorectal cancer/neoplasm, as well as with the search terms: alcohol and tobacco consumption and cancer/neoplasms of the UADT or head and neck cancer. The search was limited to human studies, the publication type meta-analysis as well as papers written in English or German. To assess the quality of the identified meta-analyses, the AMSTAR-Score [18] was used, which is a tool to assess the methodological quality of systematic reviews by an 11-item questionnaire.
Meta-analyses were eligible for inclusion if they reported risk estimates for incident cancer according to the defined ICD-10 codes and categories for alcohol drinking in g/d or drinks/d (and tobacco smoking in cigarettes/d for UADT cancers) and when they included studies predominantly from Europe and North America. If more than one meta-analysis was identified for inclusion, the one which reported sex-specific risk estimates, which included more studies, preferably cohort studies, over a longer period and which was judged to be of better quality according to the AMSTAR-score was used. Table 1 describes the characteristics of the selected meta-analyses. Risk estimates are presented in the supplementary tables A1 and A2 (online).
Cancer incidence in Germany
Cancer incidence in Germany for 2010 was obtained from estimates by the German Centre for Cancer Registry Data at the Robert Koch Institute. These annual national incidence calculations depend on the estimated completeness of each registry in the network providing nationwide coverage. Completeness estimates are based on mortality/incidence ratios and five well-established registries fulfilling defined quality criteria as a reference region [19]. For 2010, the estimated total number of incident cancer cases of 477,300 are based on 429,866 notified cases transmitted by the registries at the end of 2012. Approximately 156,000 incident cases were estimated for alcohol-related cancers in the German population aged ≥35 years. Age-, sex-, and site-specific incidence rates were estimated using these data.
Statistical analysis
We applied the method from Tseng et al. [20] to calculate PARs. This method expands upon the formula for use with case–control data by Bruzzi et al. [21] so that it may be used with confounder-adjusted risk estimates from meta-analyses and prevalence estimates from survey data. For the purposes of PAR calculations, age at diagnosis was considered to be the sole confounder and was categorized into five groups (35–44, 45–54, 55–64, 65–74, 75+ years). As per Bruzzi et al. [21], the PAR can be estimated according to:
where p (c)i represents the proportion of cases in the ith of k + 1 exposure levels and RR i the confounder-adjusted risk of the outcome at the ith exposure level relative to the risk in the reference category (i = 0; zero exposure) (for levels of alcohol and tobacco associated with RR i see supplementary tables A1, A2). The RR i were derived from published meta-analyses, as mentioned above, and p (c)i , the proportion of cases at the ith level of exposure, can be estimated by
where p ij is the proportion of men or women in exposure category i and confounder level j in the general population. RR ij represents the risk of those in that exposure-age category ij and can be estimated as follows, with the assumption that there is no interaction on the multiplicative scale between exposure and confounder:
where RR i is the confounder- (age-) adjusted exposure effect and RR *j the exposure-adjusted confounder effect. As mentioned above, the age-adjusted exposure effect (RR i ) was taken from meta-analyses, whereas the exposure-adjusted age effect was calculated using the following formula:
where R (w)j is the estimated cancer incidence rate for age group j and \(p_{j} = \sum\nolimits_{i = 0}^{k} {p_{ij} ,}\) or the total proportion of the population in age group j in the general population.
To examine how uncertainty in the relative risks could be reflected in our PAR estimates, we simulated 3,000 sets of risk estimates assuming independent log-normal distributions based on the published point estimates and the standard errors derived from published 95 % confidence intervals. Due to the complex sampling design of the DEGS1 survey, a fully appropriate resampling technique with which we could incorporate uncertainty in the risk factor distribution was not identified. However, the bootstrap approach described by Canty and Davison [22] and implemented in the R package “survey” [23] in combination with the aforementioned relative risk simulations resulted in intervals that were only slightly wider than those incorporating solely the relative risk simulations. Therefore, we present the 2.5th and 97.5th percentile PAR estimates calculated with simulated RR i and the point estimates of the risk factor distributions. For esophageal cancer, simulations could not be conducted, because confidence intervals for the relative risks were not published for the exposure-specific analysis. Nonetheless, the selected analysis on esophageal cancer was the most appropriate we identified.
Absolute numbers of attributable cases in 2010 were calculated using the resulting PARs and estimated national cancer incidence from this year [24]. For calculating the total absolute number of attributable cases, two procedures were followed: (1) all absolute numbers, also negative ones, were included and (2) only those absolute numbers were summed for which the 2.5th–97.5th percentile range of the PAR did not include 1.0.
PAR values were estimated for all considered cancer types assuming the total elimination of alcohol consumption in the population (reference category of zero exposure). PAR estimates regarding UADT cancers are presented for the effect of alcohol elimination by different levels of tobacco smoking. To obtain appropriate RRs for these stratified analyses, published RRs of the joint interaction effects were divided by the RR of the corresponding reference category for alcohol consumption (see supplementary table A1).
Results
Risk factor prevalence analyses are based on data from 2,919 men and 3,007 women with an average age of 54 (SD 11.9) and 55 (SD 12.3) years, respectively. With approximately 80 %, moderate drinking was most prevalent among men and women in Germany, only 10 % of men and 2.0 % of women reported being heavy drinkers. Among men, there were 27.3 % current and 40.4 % former smokers. Among women, 23.2 % were current and 25.5 % former smokers. The highest alcohol consumption category (≥3 drinks/day) was approximately twice as frequent among smokers as among non-smokers (Table 4).
Table 2 presents overall PAR estimates for alcohol consumption among German adults in 2010. The greatest PAR for alcohol consumption was estimated for esophageal cancer (men: 47.6 %, women: 35.8 %), followed by pharyngeal and laryngeal cancers. PARs for colorectal cancer in men were 9.7 % (95 % CI 3.6–15.8) and for breast cancer in women 6.6 % (95 % CI 4.9–8.4). Negative PAR estimates were observed for liver cancer (men: −4.9 %, 95 % CI −26.6 to 15.5; women: −12.1 %, 95 % CI −30.8 to 5.0) as well as for colorectal cancer among women (−2.9 %, 95 % CI −7.6 to 1.5). These results were due to negative—though non-significant—relative risk estimates.
We estimate that approximately 8,700 cancer cases among men and 4,900 cases among women diagnosed in 2010 could be attributable to alcohol consumption in Germany. More than 90 % of the cases among women are breast cancer cases. Among men, the largest absolute number was estimated for colorectal cancer, despite the comparatively small PAR.
Tables 3, 4 and 5 show risk factor prevalence and PAR by exposure category. Moderate drinking (<3 drinks per day) is most prevalent in Germany. For colon-rectum and breast, this exposure category also constitutes the highest PAR. For UADT cancers, for which alcohol and tobacco are synergistically interacting risk factors, moderate consumption of alcohol among non-smokers contributes only a small amount to the overall PAR. On the contrary, moderate consumption of alcohol together with tobacco smoking constitutes the greatest PAR among women (Tables 4, 5). Consumption of ≥3 drinks per day among smokers, although uncommon, contributes considerably to the overall PAR of oral cavity and pharyngeal cancer among men.
Discussion
Our analysis indicates that approximately 3 % of all incident cancer cases (excluding non-melanoma skin cancer) among German adults aged ≥35 years could be attributable to alcohol consumption in 2010. This corresponds to approximately 12,900 potentially preventable cases annually (up to 13,600 cases if only significant PAR were considered). The highest proportion of alcohol-attributable cancer cases is estimated for esophageal cancer with 47.6 % among men and 35.8 % among women. However, the greatest absolute numbers of attributable cases were estimated for breast cancer (4,500 cases among women) and colorectal cancer (3,200 cases among men).
Moderate alcohol consumption contributes substantially to the overall PAR of breast and colorectal cancer, which is mainly due to the high prevalence of this exposure category. Regarding cancers of the UADT, moderate alcohol consumption among non-smokers is also highly prevalent (especially among women), but contributes only a small amount to the overall PAR. In contrast, moderate-to-heavy consumption of alcohol among smokers contributes a considerable proportion to the overall PAR due to synergistic interactions. Thus, a substantial potential for reducing UADT cancer incidence lies in the elimination or reduction in drinking among smokers.
Other analyses of the population-level impact of alcohol consumption on cancer incidence in Germany haven been published previously. A current analysis by the EPIC study group [7] reported somewhat higher PARs for UADT and colorectal cancer than our analyses. The PAR for breast cancer (7 %) is very similar to our estimate. The results for liver cancer differ considerably, which may be due to higher alcohol consumption estimates (based on survey data combined with sales data) and higher relative risks in the EPIC study. However, PAR estimates for liver cancer also differ considerably between other previous European studies [25–27], due to differences in the assessment of alcohol consumption prevalence and relative risk estimates.
John and Hanke [8] estimated the cancer mortality attributable to alcohol and tobacco in Germany in the 1990s. Overall, they found that only 0.2 % of cancer deaths were attributable to alcohol alone and a further 5.6 % to alcohol and tobacco consumption. Due to differences in exposure prevalence and endpoint (incidence vs. mortality), a direct comparison with our estimates would be inappropriate. Two studies using case–control data from Europe and America [5, 6] reported largely similar PARs for alcohol consumption among never smokers for UADT cancers. However, interpretability of these results is also limited because of the different methods used, especially regarding exposure definitions.
Limitations and strengths
PAR estimates depend on the chosen exposure definition and validity of exposure assessment. Alcohol consumption could be underreported in the self-administered FFQ of the DEGS1 study. Although studies investigating reproducibility and validity of self-reported alcohol drinking in different populations have found adequate conformity [28, 29], underreporting of socially undesirable responses in self-reported data should not be ruled out. Moreover, it seems likely that heavy drinkers and alcohol-addicted persons are underrepresented in the survey. Both underreporting and underrepresented heavy drinkers would lead to an underestimation of our PAR.
In our analyses, we neither considered lag time nor changes in individual drinking or smoking behavior, simplifications that may not reflect the true carcinogenic effects of these risk factors, which are as yet incompletely understood. Regarding alcohol consumption, it has been suggested that the intensity of drinking may be more important for cancer risk than the duration alone [1, 4]. Drinking many drinks per day for a short duration has been shown to be more harmful than drinking fewer drinks per day for a longer duration [30]. The survey data we used to derive prevalence estimates only considered current alcohol consumption habits, without considering past consumption patterns that may have differed in intensity from current ones. If former drinkers still have an elevated risk for cancer and are therefore misclassified as unexposed, our results may underestimate actual PARs.
PAR estimates strongly depend on accurate relative risk estimates and hence on our selection of meta-analyses. Considerable uncertainty exists concerning the association between alcohol consumption and liver cancer risk, especially for moderate drinking. One factor complicating the quantification of this association is the occurrence of alcohol-related diseases, such as cirrhosis, that influence both alcohol consumption habits as well as liver cancer risk. Such diseases can lead to a substantial decrease in alcohol consumption [31], an increase in liver cancer risk [32], and in turn to an underestimation of the association between alcohol consumption and liver cancer. These uncertainties may be a source of heterogeneity between studies of alcohol consumption and liver cancer, leading to different estimates of attributable risks [7, 17, 25]. Our PAR estimates for liver cancer are mainly based on negative (though non-significant) risk estimates for moderate drinking. Had we only considered heavy drinking, with relative risk estimates >1.0, we would have estimated that 4.4 % of liver cancers could have been attributable to alcohol consumption in 2010.
For esophageal cancer, the study from Castellsagué et al. [14] only reported RR estimates for men, which we then applied to the calculations for women. This might result in an underestimation of the PAR for women, as other studies found a higher alcohol-associated esophageal cancer risk among women [33, 34].
Similar to liver cancer, the negative PAR for moderate alcohol drinking among non-smokers in oral cavity cancer should be interpreted with caution. The INHANCE pooled analysis [6] found a slight but non-significant decreased risk of oral cavity cancer among never smokers/light drinkers. People in this exposure category comprise a large proportion of the German population, and the PAR calculations resulted in a negative estimate (Table 4; Supplementary Table A1). This should not be interpreted as a ‘protective’ effect of alcohol consumption. Rather, it suggests that alcohol consumption alone, without concurrent tobacco consumption, has little to no effect on oral cavity cancer risk.
In summary, the limitations described above may have resulted in conservative PAR estimates. Although these PAR estimates are based on the assumption of complete elimination of alcohol consumption and thus might be unrealistic to attain in practice, they represent the theoretical preventive potential and may provide a helpful basis for decision making in public health.
The current analysis presents the proportion of incident cases that could be attributed to alcohol consumption in Germany in 2010 on the basis of nationally representative prevalence estimates from the DEGS1 study. These PAR estimates are likely to be more applicable to the broader population than estimates based on prevalence data from regional studies. Furthermore, these prevalence estimates allowed the consideration of the joint exposure to alcohol and tobacco, which is relevant when evaluating cancers of the UADT. Moreover, a much more comprehensive view of disease burden is given by focusing on cancer incidence as opposed to mortality only. As the DEGS surveys in Germany are performed regularly, we plan to update PAR estimates periodically in order to analyze time trends.
This paper focuses on cancers for which alcohol is an independent risk factor (colorectal, liver, breast) or for which alcohol interacts synergistically with tobacco smoking (UADT cancer). This represents only a part of the overall burden of disease associated with alcohol consumption. The high burden of alcohol-associated diseases other than cancer should be considered as well, since cancer prevention is not the only benefit to be expected when eliminating or reducing alcohol consumption. For example, more than 2,200 children with fetal alcohol syndrome are born annually in Germany [35]. Decreased alcohol consumption would reduce the occurrence of this entirely alcohol-attributable disease as well as other widespread diseases like liver cirrhosis, cardiovascular diseases or injuries, and accidents due to alcohol consumption.
Conclusion
Some of the most common and lethal cancers in Germany are attributable to alcohol. Many of the estimated cases are attributable to the moderate consumption of alcohol or to alcohol consumption with concurrent tobacco smoking (UADT cancers). In addition, the relatively small proportion of male smokers with high levels of alcohol consumption also contributes a large proportion of oral cavity and pharyngeal cancers. A reduction in both moderate and heavy drinking provides a substantial preventive potential. A further reduction in tobacco consumption would yield an even greater reduction in cancers of the UADT due to synergistic interactions of these exposures. In summary, our results demonstrate the impact of alcohol across different consumption levels on cancer incidence in Germany, suggesting that public health interventions should address exposure at all levels.
Abbreviations
- AMSTAR:
-
Assessment of multiple systematic reviews
- DEGS1:
-
German Health Interview and Examination Survey for Adults (2008–2011)
- FFQ:
-
Food frequency questionnaire
- IARC:
-
International Agency for Research on Cancer
- PAR:
-
Population attributable risk
- RKI:
-
Robert Koch Institute
- RR:
-
Relative risk
- UADT:
-
Upper aerodigestive tract
References
IARC (2012) A review of human carcinogens. Part E: personal habits and indoor combustions. In: World Health Organization, International Agency for Research on Cancer (eds) IARC monographs on the evaluation of carcinogenic risks to humans. World Health Organization, Lyon
Boffetta P, Hashibe M (2006) Alcohol and cancer. Lancet Oncol 7:149–156
Wight AJ, Ogden GR (1998) Possible mechanisms by which alcohol may influence the development of oral cancer—a review. Oral Oncol 34:441–447
Pelucchi C, Gallus S, Garavello W, Bosetti C, Vecchia CL (2008) Alcohol and tobacco use, and cancer risk for upper aerodigestive tract and liver. Eur J Cancer Prev 17:340–344
Anantharaman D, Marron M, Lagiou P et al (2011) Population attributable risk of tobacco and alcohol for upper aerodigestive tract cancer. Oral Oncol 47:725–731
Hashibe M, Brennan P, Chuang S et al (2009) Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the international head and neck cancer epidemiology consortium. Cancer Epidemiol Biomark Prev 18:541–550
Schütze M, Boeing H, Pischon T et al (2011) Alcohol attributable burden of incidence of cancer in eight European countries based on results from prospective cohort study. BMJ 342:d1584
John U, Hanke M (2002) Tobacco smoking- and alcohol drinking-attributable cancer mortality in Germany. Eur J Cancer Prevention. 11:11–17
Lampert T, von der Lippe E, Müters S (2013) Prevalence of smoking in the adult population of Germany. Results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 56:802–808
Gaertner B, Meyer C, John U, Freyer-Adam J (2013) Alkohol - Zahlen und Fakten zum Konsum. In: Deutsche Hauptstelle für Suchtfragen e.V. (ed) Jahrbuch Sucht 2013. Pabst, Hamm
Hamajima N, Cancer CGoHFiB (2002) Alcohol, tobacco and breast cancer—collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer 87:1234–1245
Scheidt-Nave C, Kamtsiuris P, Gößwald A et al (2012) German Health Interview and Examination Survey for Adults (DEGS)—design, objectives and implementation of the first data collection wave. BMC Public Health 12:730
Haftenberger M, Heuer T, Heidemann C, Kube F, Krems C, Mensink GB (2010) Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr J 9:36
Castellsagué X, Munoz N, De Stefani E et al (1999) Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer 82:657–664
Fedirko V, Tramacere I, Bagnardi V et al (2011) Alcohol drinking and colorectal cancer risk: an overall and dose–response meta-analysis of published studies. Ann Oncol 22:1958–1972
Ridolfo B, Stevenson C (2001) The quantification of drug-caused mortality and morbidity in Australia, 1998. In: Australian Institute of Health and Welfare (ed) Drug statistics series. Australian Institute of Health and Welfare, Canberra
Turati F, Galeone C, Rota M et al (2014) Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Ann Oncol 25:1526–1535
Shea BJ, Grimshaw JM, Wells GA et al (2007) Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 7
Kraywinkel K, Barnes B, Dahm S, Haberland J, Nennecke A, Stabenow R (2014) Nationwide statements from regional data. Methods of the Center for Cancer Registry Data. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 57:13–21
Tseng M, Weinberg CR, Umbach DM, Longnecker MP (1999) Calculation of population attributable risk for alcohol and breast cancer (United States). Cancer Causes Control 10:119–123
Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C (1985) Estimating the population attributable risk for multiple risk factors using case–control data. Am J Epidemiol 122:904–914
Canty AJ, Davison AC (1999) Resampling-based variance estimation for labour force surveys. J R Stat Soc Ser D (Stat) 48:379–391
Lumley T (2009) Analysis of complex survey samples. R-package “survey”, 3.30-3 edn. R Foundation, Vienna
RKI (2013) Cancer in Germany 2009/2010. Robert Koch Institute and the Association of Population-based Cancer Registries in Germany, Berlin
Trichopoulos D, Bamia C, Lagiou P et al (2011) Hepatocellular carcinoma risk factors and disease burden in a European cohort: a nested case–control study. J Natl Cancer Inst 103:1686–1695
Parkin DM (2011) Cancers attributable to consumption of alcohol in the UK in 2010. Br J Cancer 105:S14–S18
Eliasen M, Becker U, Gronbaek M, Juel K, Schurmann TolstrupJ (2014) Alcohol-attributable and alcohol-preventable mortality in Denmark: an analysis of which intake levels contribute most to alcohol’s harmful and beneficial effects. Eur J Epidemiol 29:15–26
Ferraroni M, Decarli A, Franceschi S et al (1996) Validity and reproducibility of alcohol consumption in Italy. Int J Epidemiol 25:775–782
Giovannucci E, Colditz GA, Stampfer MJ et al (1991) The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol 133:810–817
Lubin JH, Purdue M, Kelsey K et al (2009) Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: a pooled analysis of case–control studies. Am J Epidemiol 170:937–947
Grewal P, Viswanathen VA (2012) Liver cancer and alcohol. Clin Liver Dis 16:839–850
La Vecchia C, Negri E, Cavalieri d’Oro L, Franceschi S (1998) Liver cirrhosis and the risk of primary liver cancer. Eur J Cancer Prev 7:315–320
Bagnardi V, Blangiardo M, La Vecchia C, Corrao G (2001) Alcohol consumption and the risk of cancer. Alcohol Res Health 25:263–270
Weikert C, Dietrich T, Boeing H et al (2009) Lifetime and baseline alcohol intake and risk of cancer of the upper aero-digestive tract in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Intl J Cancer 125:406–412
Singer MV, Teyssen S (2001) Alkoholassoziierte Organschäden. Befunde in der Inneren Medizin, Neurologie und Geburtshilfe/Neonatologie. Dtsch Ärztebl 98:A2109–A2120
Acknowledgments
The authors thank all the German cancer registries as well as the teams of the German health survey at the Robert Koch Institute for providing the comprehensive data sets.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wienecke, A., Barnes, B., Neuhauser, H. et al. Incident cancers attributable to alcohol consumption in Germany, 2010. Cancer Causes Control 26, 903–911 (2015). https://doi.org/10.1007/s10552-015-0566-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-015-0566-8