Abstract
Purpose
Breast cancer mortality is higher in Black women than other racial groups. This difference has been partially attributed to a higher proportion of triple-negative breast cancer (TNBC). However, it is uncertain if survival disparities exist in racially diverse TNBC patients receiving similar treatments. Here, we examine racial differences in disease-related outcomes in TNBC patients treated on the E5103 clinical trial.
Methods
From 2007 to 2011, 4,994 patients with stage I-III HER2-negative breast cancer were randomized to adjuvant chemotherapy with or without bevacizumab. This analysis was limited to the subset of 1,742 TNBC patients with known self-reported race. Unadjusted Kaplan-Meier curves and adjusted Cox-Proportional Hazards models were used to determine breast cancer events and survival outcomes.
Results
Of the analysis population, 51 (2.9%) were Asian, 269 (15.4%) Black, and 1422 (81.6%) White. Median age was 51 years. Patient characteristics, treatment arm, and local therapies were similar across racial groups. White women were more commonly node-negative (56% vs. 49% and 44% in Asian and Black women, respectively; p < 0.01). At a median follow-up of 46 months, unadjusted Kaplan-Meier locoregional and distant recurrence, and disease-free and overall survival, did not differ significantly by race. In Cox models adjusted for patient and tumor characteristics and treatment arm, race was not associated with any disease event. Larger tumor size and nodal involvement were consistently associated with breast cancer events.
Conclusion
This clinical trial population of similarly treated TNBC patients showed no racial differences in breast cancer outcomes. Disease extent, rather than race, was associated with disease events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite advances in breast cancer screening and treatments, breast cancer mortality rates remain higher for non-Hispanic Black (NHB) patients compared to non-Hispanic White (NHW) patients [1], with 40% increased mortality among NHB patients across breast cancer subtypes. Triple-negative breast cancer (TNBC) represents a diverse group of aggressive cancers defined by lack of estrogen, progesterone, and human epidermal growth factor 2 (HER2) receptors. TNBC is more common in NHB patients, comprising up to 21% of breast cancer diagnoses in 2020 as compared to 11% of diagnoses in NHW patients [2]. Further, TNBC tends to present at a younger age and at a more advanced disease stage in NHB patients [3, 4].
Among patients with TNBC within national cancer registry data, NHB patients have been noted to have 7% higher breast cancer mortality and 4% higher all-cause mortality compared to NHW patients [1, 2, 5]. These findings may be partially explained by presentation at more advanced stages of disease, earlier age at diagnosis, and more aggressive tumor biology compared to NHW patients [6, 7]. Additionally, there is increasing evidence that stress secondary to experienced racism and poverty may mediate the increased mortality from TNBC [8]. Thus, some of the racial disparities in TNBC outcomes noted in the general population may be confounded by various social determinants of health including limited healthcare access or differential receipt of treatment [4, 9].
Although there appear to be disparate survival outcomes by race in patients with TNBC in population-based cancer registry studies, it is not clear whether these differences persist within clinical trial populations. Provided that certain non-biological factors are controlled for, the clinical trial setting can be helpful to determine whether racial disparities persist in an environment with more uniform access to care and treatment. The purpose of this study was to explore disease outcomes by race in patients with TNBC treated with adjuvant chemotherapy in the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network (ECOG-ACRIN) E5103 clinical trial.
Methods
Data source
E5103 was a double-blind randomized phase III clinical trial that examined the effect of adjuvant chemotherapy with or without bevacizumab on invasive disease-free survival (IDFS) in patients with HER2-negative breast cancer. Between 2007 and 2011, 4,994 patients with node-positive or high-risk node-negative stage I-III HER2-negative disease were enrolled. Patients were randomized to adjuvant chemotherapy with or without bevacizumab. Eligible patients with TNBC included those with tumors > 1 cm or with node-positive disease. Patients were excluded if they had any prior cytotoxic chemotherapy or hormonal therapy, major surgery within 4 weeks of protocol start, and/or inadequate hepatic, renal, or hematologic function. The full study criteria have previously been reported [10]. All patients were treated with adjuvant doxorubicin and cyclophosphamide (AC) every 2–3 weeks for 4 cycles followed by paclitaxel (T) weekly for 12 weeks. Patients were randomized to either chemotherapy alone (arm A), chemotherapy with concurrent bevacizumab (arm B), or chemotherapy with concurrent and sequential bevacizumab (arm C). Randomization schema and inclusion criteria for the parent and present sub-study are outlined in Fig. 1.
Variables and endpoints
This study was an unplanned, secondary analysis of the prospective E5103 clinical data and was limited to the 1,742 patients with TNBC and known self-reported race. Exclusions included 3228 patients with hormone-receptor positive tumors, 20 patients without known self-reported race, 3 with missing tumor size, and 1 patient with no follow-up information (Fig. 1). Available patient characteristics included age, race, and sex, and ECOG functional status. Cancer-specific variables included tumor size, nodal status, histologic grade, lymph node involvement, and performance status. Treatment metrics such as primary surgery and local therapy, follow-up time, adverse events, and treatment arm were also included.
The endpoints for this post hoc analysis were disease events, namely locoregional recurrence (LRR), distant recurrence (DR), IDFS, and overall survival (OS). Follow-up data were collected from the time of randomization to time of event or last follow-up. LRR was defined as ipsilateral recurrence in breast, skin, chest wall, or regional nodes without concurrent DR; patients were censored at the time of a non-LRR event. DR was defined as a disease recurrence outside of the breast or ipsilateral regional nodal basin; patients were censored at the time of a non-DR event. IDFS was defined as time from the date of randomization to the first treatment failure (invasive ipsilateral, local/regional, or distant recurrence, invasive contralateral breast cancer, invasive non-breast second primary malignancy or death from any cause); cases without documented IDFS event were censored at the date of last follow-up. OS was defined as the time from date of randomization to death from any cause; otherwise, cases were censored at date last follow-up.
Statistical analysis
Descriptive statistics were used to examine patient, tumor, and treatment characteristics by race. Unadjusted Kaplan-Meier hazard curves were used to estimate 4-year LRR, DR, IDFS, and OS by race. A Cox proportional hazards regression analysis, adjusted for patient, tumor, and treatment factors, was used to identify hazard of each disease event. Covariates included race, study arm, tumor size, lymph node involvement, primary surgery, and radiation therapy. Model variables were chosen a priori. Hazard ratios (HR) > 1 suggested a higher hazard of disease event. All Confidence Intervals (CIs) are reported at a 95% level of significance. All P-values were 2 sided and P < 0.05 was considered statistically significant. SAS v 9.4 was used for all analyses.
Results
Cohort characteristics
A total of 1,742 patients with stage I-III TNBC enrolled in ECOG-ACRIN-E5103 trial were included in this analysis. Of these patients, 1,422 (81.6%) were White, 269 (15.4%) were Black, and 51 (2.9%) were Asian. Cohort characteristics are described in Table 1. The mean age was 51 years (range 21–82). Most patients had T2 (n = 921, 52.9%) or T3 tumors (n = 98, 5.6%) and just over half had node-negative disease (n = 941, 54.0%). Most patients had grade 3 disease (n = 1714, 88.5%). 909 patients (52.2%) underwent breast conservation and 833 (47.8%) underwent mastectomy.
Age, treatment arm, and locoregional treatment receipt were similar across racial groups (Table 1). White women were more commonly node-negative (56.1%) compared to Black (44.2%) and Asian women (49.0%), p = 0.005. Anatomic stage differed across racial groups, with White women having more stage I disease (25.6%), compared to 16.7% of Black women and 21.6% of Asian women (p = 0.032). Tumor size and grade were evenly distributed across racial groups.
Disease events by race
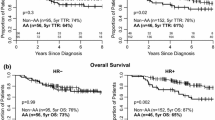
At a median follow-up of 46 months, the crude incidence of disease events did not differ significantly by race. Across all racial subgroups, LRR ranged from 2.0 to 8.6% (p = 0.281), DR ranged from 10.8 to 17.7% (p = 0.348), any IDFS event ranged from 17.7 to 20.5% (p = 0.895), and any death ranged from 9.8 to 12.7% (p = 0.595). Unadjusted 4-year Kaplan-Meier estimates of LRR, DR, IDFS, and OS were not significantly different by racial group (Fig. 2).
Although there were numerical differences in LRR and DR, these did not reach statistical significance: the estimated 4-year LRR rate was 8% for White patients, 11% for Black patients, and 2% for Asian patients (p = 0.230) and the 4-year DR rate was 14%, 12%, and 19% for White, Black, and Asian patients, respectively (p = 0.410).
In Cox-Proportional Hazards models adjusted for patient, treatment, and tumor factors, race was not associated with any disease event or survival (Table 2). Increasing tumor size and nodal involvement were significantly associated with all disease events and survival. T3 (vs. T1) tumor size was associated with 2-5-fold increased hazard across disease outcomes. Node-positivity was also associated with an increased hazard for each disease outcome. Mastectomy with postmastectomy radiation (compared to breast-conserving surgery with radiation) was associated with decreased LRR events and mastectomy alone was associated with increased IDFS and OS events. Age ≥ 50 was associated with decreased DR events and not associated with other outcomes.
Discussion
In this post-hoc analysis of TNBC patients treated with adjuvant chemotherapy +/- bevacizumab on the E5103 clinical trial, there were no differences observed in LRR, DR, IDFS, or OS, by race in either unadjusted or adjusted analyses. Disease extent, characterized by tumor size and nodal burden, were the primary factors associated with breast cancer events and survival.
Population-based and multi-institutional studies generally suggest an increased incidence of and mortality from TNBC in Black women compared to White women [3, 11,12,13,14,15], while single institutional series are more varied in terms of whether racial disparities are present in survival outcomes of TNBC [16,17,18]. These studies frequently demonstrate advanced stage of disease at diagnosis, earlier onset of cancer, and more aggressive tumor biology in NHB patients compared to NHW patients [6, 7]. Although biological factors are commonly cited as an explanation for the racial differences in mortality from TNBC, mortality differences are not fully explained by biologic factors alone, and the role of social determinants of health is an increasingly recognized contributing factor to disparities [14, 19].
Several population-based studies have demonstrated that structural racism leading to neighborhood disadvantage contributes to the increased mortality seen in Black women with TNBC [9, 20]. Further, studies have shown that NHB women are more likely to have public or no insurance, diminishing access to care and increasing incidence of and mortality from.
TNBC [5, 8, 21]. Even when NHB women have access to care, they have lower odds of receiving treatment for TNBC compared to their White counterparts, a finding that has been associated with increased mortality [4, 12]. NHB women are also more likely to have delayed initiation of treatment after diagnosis of TNBC [5, 22]. Taken together, the literature suggests that diminished access to care, decreased uptake of and delays in treatment within the NHB population contribute to survival disparities in TNBC.
Clinical trial populations represent a select patient population with the support, willingness, and access to participate in clinical trials. Previous work examining inequities in clinical trial populations has suggested that disparities in outcomes by race still exist in hormone-receptor positive breast cancer, while this effect appears to be attenuated in hormone-receptor negative breast cancer [23,24,25,26]. In a retrospective analysis of 6676 patients in 35 Southwest Oncology Group trials, post-menopausal Black women with hormone receptor positive breast cancer were found to have higher mortality than other postmenopausal patients, but this difference was not observed in premenopausal patients with hormone receptor-negative breast cancer [23]. Similarly, a pooled analysis of 9702 patients in 8 National Surgical Adjuvant Breast and Bowel Project trials demonstrated that Black race was associated with inferior distant relapse-free survival in hormone receptor-positive breast cancer but not in hormone receptor- negative breast cancer [27]. Within the E1199 randomized clinical trial comparing the efficacy of different taxane regimens in patients with node-positive, stage II-IIIA breast cancer, a secondary analysis of 4817 eligible patients demonstrated that Black patients with hormone receptor- positive breast cancer had worse disease-free and OS compared to non-Black patients, but this was not observed in the subset of patients with TNBC [28]. A previous correlative analysis of the E5103 clinical trial focused on genetic ancestry differences in treatment toxicity and outcomes, also finding a trend of inferior disease-free survival in patients of African ancestry with hormone receptor-positive disease but not in patients with hormone receptor-negative breast cancer [29]. Consistent with these previous analyses in clinical trial populations, our study did not find differences in IDFS or OS in patients with TNBC in the E5103 trial; further, it demonstrated no differences in LRR or DR between racial groups.
It is interesting that racial disparities appear to exist differentially across subtypes of breast cancer, even within clinical trial populations. One possible explanation is that within TNBC, treatment is more standardized to include locoregional therapy and chemotherapy in most patients. In hormone receptor-positive breast cancer, longer-term treatments, including extended duration adjuvant endocrine therapy, allow more opportunities for differential access, adherence, tolerance, duration of treatments, and possibly differential endocrine resistance mechanisms, all of which could contribute to disparate outcomes [30,31,32,33,34]. Outside of a clinical trial population, mortality differences may be prevalent across all breast cancer subtypes due to the increased influence of social determinants of health in the general population and disparities across the care continuum [8, 20, 26, 35]. Although our findings for a lack of disparities in the current study are encouraging, the lack of differences in disease outcomes by race may be related to the inherent selection bias of patients willing and able to participate in a clinical trial, which may account for less variation in socioeconomic factors that disproportionately affect Black women with TNBC. This study underscores the importance of accruing diverse patient populations onto clinical trials so that treatment results can be meaningfully generalized to the overall patient population.
Additional studies are warranted to further address the question of whether race-related variation exists in TNBC biology, especially as novel immunotherapy treatments become available. Existing studies demonstrate differences in the immune landscape of breast cancer between Black and White women as well as in TNBC subtypes [36,37,38]. These differences may become clinically relevant as advances are made in therapeutic manipulation of the tumor microenvironment.
This work has several limitations. Importantly, the trial was not powered for analysis of disease outcomes by race, and small cohorts of NHB and Asian patients limit analyses. As the trial was not designed with LRR and DR in mind, additional adjustment for potential confounding factors such as margin status and germline mutation status were not available. The socioeconomic status of the patients was also unavailable. Lastly, there is an inherent selection bias within clinical trial populations that limits generalizability to the general population. Despite these limitations, the strengths of this study include the use of a large, multicenter, randomized, clinical trial which was open at sites throughout the United States, providing a robust cohort to examine disparities and allows comparison of patients receiving similar treatments.
Conclusions
In this post-hoc analysis of the randomized E5103 clinical trial, there were no racial disparities in LRR, DR, IDFS or OS. Additional data are needed to better understand the disparities observed outside of a clinical trial setting. Our findings for similar outcomes within the context of relatively uniformly treated cohort raise the question of how much of the disparities seen outside of a clinical trial are related to differences in access and treatment.
Data availability
The datasets generated during and/or analyzed during the current study are available on request from the NCTN Data Archive [https://nctn-data-archive.nci.nih.gov/].
References
National Cancer Institute Surveillance, Epidemiology, and End Results Program Cancer Stat Facts: Female Breast Cancer. https://seer.cancer.gov/statfacts/html/breast-subtypes.html. Accessed September 9th, 2023
SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute (2023) Apr 19. [updated: 2023; cited 2023 Sep 9]. https://seer.cancer.gov/statistics-network/explorer/. Data source(s): SEER Incidence Data, November 2022 Submission (1975–2020), SEER 22 registries
Zhang W, Bai Y, Sun C, Lv Z, Wang S (2022) Racial and regional disparities of triple negative breast cancer incidence rates in the United States: an analysis of 2011–2019 NPCR and SEER incidence data. Front Public Health 10:1058722. https://doi.org/10.3389/fpubh.2022.1058722
Cho B, Han Y, Lian M, Colditz GA, Weber JD, Ma C, Liu Y (2021) Evaluation of Racial/Ethnic Differences in Treatment and mortality among women with triple-negative breast Cancer. JAMA Oncol 7(7):1016–1023. https://doi.org/10.1001/jamaoncol.2021.1254
Wang F, Zheng W, Bailey CE, Mayer IA, Pietenpol JA, Shu XO (2021) Racial/Ethnic disparities in all-cause mortality among patients diagnosed with triple-negative breast Cancer. Cancer Res 81(4):1163–1170. https://doi.org/10.1158/0008-5472.CAN-20-3094
Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL (2018) Racial and ethnic disparities in Cancer Survival: the contribution of Tumor, Sociodemographic, Institutional, and Neighborhood characteristics. J Clin Oncol 36(1):25–33. https://doi.org/10.1200/JCO.2017.74.2049
Jemal A, Robbins AS, Lin CC, Flanders WD, DeSantis CE, Ward EM, Freedman RA (2018) Factors that contributed to Black-White disparities in Survival among Nonelderly Women with breast Cancer between 2004 and 2013. J Clin Oncol 36(1):14–24. https://doi.org/10.1200/JCO.2017.73.7932
Prakash O, Hossain F, Danos D, Lassak A, Scribner R, Miele L (2020) Racial disparities in Triple negative breast Cancer: a review of the role of Biologic and non-biologic factors. Front Public Health 8:576964. https://doi.org/10.3389/fpubh.2020.576964
Eldridge L, Berrigan D (2022) Structural racism and triple-negative breast Cancer among Black and White women in the United States. Health Equity 6(1):116–123. https://doi.org/10.1089/heq.2021.0041
Miller KD, O’Neill A, Gradishar W, Hobday TJ, Goldstein LJ, Mayer IA, Bloom S, Brufsky AM, Tevaarwerk AJ, Sparano JA, Le-Lindqwister NA, Hendricks CB, Northfelt DW, Dang CT, Sledge GW Jr. (2018) Double-blind phase III trial of Adjuvant Chemotherapy with and without Bevacizumab in patients with lymph node-positive and high-risk lymph node-negative breast Cancer (E5103). J Clin Oncol 36(25):2621–2629. https://doi.org/10.1200/JCO.2018.79.2028
Makhani SS, Bouz A, Stavros S, Zucker I, Tercek A, Chung-Bridges K (2022) Racial and ethnic inequality in Survival outcomes of Women with Triple negative breast Cancer. Cureus 14(7):e27120. https://doi.org/10.7759/cureus.27120
Oppong BA, Rolle AA, Ndumele A, Li Y, Fisher JL, Bhattacharyya O, Adeyanju T, Paskett ED (2022) Are there differences in outcomes by race among women with metastatic triple-negative breast cancer? Breast Cancer Res Treat 196(2):399–408. https://doi.org/10.1007/s10549-022-06736-8
Sung H, Wiese D, Jatoi I, Jemal A (2023) State variation in racial and ethnic disparities in incidence of Triple-negative breast Cancer among US women. JAMA Oncol 9(5):700–704. https://doi.org/10.1001/jamaoncol.2022.7835
Michaels EK, Canchola AJ, Beyer KMM, Zhou Y, Shariff-Marco S, Gomez SL (2022) Home mortgage discrimination and incidence of triple-negative and luminal A breast cancer among non-hispanic black and non-hispanic White females in California, 2006–2015. Cancer Causes Control 33(5):727–735. https://doi.org/10.1007/s10552-022-01557-y
Vidal G, Bursac Z, Miranda-Carboni G, White-Means S, Starlard-Davenport A (2017) Racial disparities in survival outcomes by breast tumor subtype among African American women in Memphis, Tennessee. Cancer Med 6(7):1776–1786. https://doi.org/10.1002/cam4.1117
Lehrberg A, Davis MB, Baidoun F, Petersen L, Susick L, Jenkins B, Chen Y, Ivanics T, Rakitin I, Bensenhaver J, Proctor E, Nathanson SD, Newman LA (2021) Outcome of African-American compared to White-American patients with early-stage breast cancer, stratified by phenotype. Breast J 27(7):573–580. https://doi.org/10.1111/tbj.14225
Sarma M, Perimbeti S, Nasir S, Attwood K, Kapoor A, O’Connor T, Early A, Levine EG, Takabe K, Kalinski P, Ambrosone C, Khoury T, Yao S, Gandhi S (2022) Lack of racial differences in clinical outcomes of breast cancer patients receiving neoadjuvant chemotherapy: a single academic center study. Breast Cancer Res Treat 192(2):411–421. https://doi.org/10.1007/s10549-021-06506-y
Cherng HR, Rice SR, Hamza M, Murali S, Rosenblatt P, Tkaczuk KHR, Bellavance E, Cheston S, Amin N, Nichols E (2021) Patterns of failure in Triple negative breast Cancer patients in an Urban, Predominately Black Population. J Racial Ethn Health Disparities 8(4):1035–1046. https://doi.org/10.1007/s40615-020-00860-1
Luo J, Kroenke CH, Hendryx M, Shadyab AH, Liu N, Chen X, Wang F, Thomas F, Saquib N, Qi L, Cheng TD, Arthur R, Wactawski-Wende J (2021) Mediation analysis of racial disparities in triple-negative breast cancer incidence among postmenopausal women. Breast Cancer Res Treat 188(1):283–293. https://doi.org/10.1007/s10549-021-06158-y
Linnenbringer E, Geronimus AT, Davis KL, Bound J, Ellis L, Gomez SL (2020) Associations between breast cancer subtype and neighborhood socioeconomic and racial composition among black and white women. Breast Cancer Res Treat 180(2):437–447. https://doi.org/10.1007/s10549-020-05545-1
Samiian L, Sharma P, Van Den Bruele AB, Smotherman C, Vincent M, Crandall M (2018) The effect of insurance and race on breast Cancer Tumor Biology and short-term outcomes. Am Surg 84(7):1223–1228
Zhang L, King J, Wu XC, Hsieh MC, Chen VW, Yu Q, Fontham E, Loch M, Pollack LA, Ferguson T (2019) Racial/ethnic differences in the utilization of chemotherapy among stage I-III breast cancer patients, stratified by subtype: findings from ten National Program of Cancer registries states. Cancer Epidemiol 58:1–7. https://doi.org/10.1016/j.canep.2018.10.015
Albain KS, Unger JM, Crowley JJ, Coltman CA Jr., Hershman DL (2009) Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst 101(14):984–992. https://doi.org/10.1093/jnci/djp175
Albain KS, Gray RJ, Makower DF, Faghih A, Hayes DF, Geyer CE, Dees EC, Goetz MP, Olson JA, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Wood WC, Keane MM, Gomez HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW, Sparano JA (2021) Race, ethnicity, and clinical outcomes in hormone Receptor-Positive, HER2-Negative, node-negative breast Cancer in the Randomized TAILORx Trial. J Natl Cancer Inst 113(4):390–399. https://doi.org/10.1093/jnci/djaa148
Abdou Y, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, Lin NU, Perez EA, Goldstein LJ, Chia SK, Dhesy-Thind S, Rastogi P, Alba E, Delaloge S, Schott AF, Shak S, Sharma P, Lew DL, Miao J, Unger JM, Tripathy D, Pusztai L, Hortobagyi GN, Kalinsky K (2023) Abstract GS1-01: Race and clinical outcomes in the RxPONDER Trial (SWOG S1007). Cancer Research 83 (5_Supplement): GS1-01-GS01-01. https://doi.org/10.1158/1538?7445.Sabcs22-gs1-01
Kantor O, King TA, Freedman RA, Mayer EL, Chavez-MacGregor M, Korde LA, Sparano JA, Mittendorf EA (2023) Racial and ethnic disparities in Locoregional recurrence among patients with hormone Receptor-Positive, node-negative breast Cancer: a Post Hoc Analysis of the TAILORx Randomized Clinical Trial. JAMA Surg 158(6):583–591. https://doi.org/10.1001/jamasurg.2023.0297
Kim G, Pastoriza JM, Qin J, Lin J, Karagiannis GS, Condeelis JS, Yothers G, Anderson S, Julian T, Entenberg D, Rohan TE, Xue X, Sparano JA, Oktay MH (2022) Racial disparity in distant recurrence-free survival in patients with localized breast cancer: a pooled analysis of National Surgical adjuvant breast and Bowel Project trials. Cancer 128(14):2728–2735. https://doi.org/10.1002/cncr.34241
Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, Perez EA, Saphner T, Wolff AC, Sledge GW Jr., Wood WC, Davidson NE (2012) Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst 104(5):406–414. https://doi.org/10.1093/jnci/djr543
Schneider BP, Shen F, Jiang G, O’Neill A, Radovich M, Li L, Gardner L, Lai D, Foroud T, Sparano JA, Sledge GW Jr., Miller KD (2017) Impact of genetic ancestry on outcomes in ECOG-ACRIN-E5103. JCO Precis Oncol 2017. https://doi.org/10.1200/PO.17.00059
Hershman D, McBride R, Jacobson JS, Lamerato L, Roberts K, Grann VR, Neugut AI (2005) Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol 23(27):6639–6646. https://doi.org/10.1200/JCO.2005.12.633
Partridge AH, Wang PS, Winer EP, Avorn J (2003) Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 21(4):602–606. https://doi.org/10.1200/JCO.2003.07.071
Wheeler SB, Spencer J, Pinheiro LC, Murphy CC, Earp JA, Carey L, Olshan A, Tse CK, Bell ME, Weinberger M, Reeder-Hayes KE (2019) Endocrine therapy nonadherence and discontinuation in Black and White women. J Natl Cancer Inst 111(5):498–508. https://doi.org/10.1093/jnci/djy136
Rauscher GH, Silva A, Pauls H, Frasor J, Bonini MG, Hoskins K (2017) Racial disparity in survival from estrogen and progesterone receptor-positive breast cancer: implications for reducing breast cancer mortality disparities. Breast Cancer Res Treat 163(2):321–330. https://doi.org/10.1007/s10549-017-4166-z
Deshmukh SK, Srivastava SK, Tyagi N, Ahmad A, Singh AP, Ghadhban AAL, Dyess DL, Carter JE, Dugger K, Singh S (2017) Emerging evidence for the role of differential tumor microenvironment in breast cancer racial disparity: a closer look at the surroundings. Carcinogenesis 38(8):757–765. https://doi.org/10.1093/carcin/bgx037
Obeng-Gyasi S, Asad S, Fisher JL, Rahurkar S, Stover DG (2021) Socioeconomic and Surgical disparities are Associated with Rapid Relapse in patients with triple-negative breast Cancer. Ann Surg Oncol 28(11):6500–6509. https://doi.org/10.1245/s10434-021-09688-3
Yao S, Cheng TD, Elkhanany A, Yan L, Omilian A, Abrams SI, Evans S, Hong CC, Qi Q, Davis W, Liu S, Bandera EV, Odunsi K, Takabe K, Khoury T, Ambrosone CB (2021) Breast Tumor Microenvironment in Black women: a distinct signature of CD8 + T-Cell exhaustion. J Natl Cancer Inst 113(8):1036–1043. https://doi.org/10.1093/jnci/djaa215
Yaghoobi V, Moutafi M, Aung TN, Pelekanou V, Yaghoubi S, Blenman K, Ibrahim E, Vathiotis IA, Shafi S, Sharma A, O’Meara T, Fernandez AI, Pusztai L, Rimm DL (2021) Quantitative assessment of the immune microenvironment in African American Triple negative breast Cancer: a case-control study. Breast Cancer Res 23(1):113. https://doi.org/10.1186/s13058-021-01493-w
Martini R, Delpe P, Chu TR, Arora K, Lord B, Verma A, Bedi D, Karanam B, Elhussin I, Chen Y, Gebregzabher E, Oppong JK, Adjei EK, Jibril Suleiman A, Awuah B, Muleta MB, Abebe E, Kyei I, Aitpillah FS, Adinku MO, Ankomah K, Osei-Bonsu EB, Chitale DA, Bensenhaver JM, Nathanson DS, Jackson L, Petersen LF, Proctor E, Stonaker B, Gyan KK, Gibbs LD, Monojlovic Z, Kittles RA, White J, Yates CC, Manne U, Gardner K, Mongan N, Cheng E, Ginter P, Hoda S, Elemento O, Robine N, Sboner A, Carpten JD, Newman L, Davis MB (2022) African Ancestry-Associated Gene expression profiles in Triple-negative breast Cancer Underlie altered Tumor Biology and clinical outcome in women of African descent. Cancer Discov 12(11):2530–2551. https://doi.org/10.1158/2159-8290.CD-22-0138
Acknowledgements
The manuscript was prepared using data from Dataset NCT00433511-D1 from the National Clinical Trials Network/National Cancer Institute (NCI) Community Oncology Research Program (NCTN/NCORP) Data Archive. Data were originally collected from clinical trial NCT number 00433511: Doxorubicin Hydrochloride, Cyclophosphamide, and Paclitaxel With or Without Bevacizumab in Treating Patients With Lymph Node-Positive or High-Risk, Lymph Node-Negative Breast Cancer. All analyses and conclusions in this manuscript are the sole responsibility of the authors and do not necessarily reflect the opinions or views of the clinical trial investigators, the NCTN, the NCORP or the NCI. EAM acknowledges support as the Rob and Karen Hale Distinguished Chair in Surgical Oncology.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by O.K., S.L. and A.J. The first draft of the manuscript was written by S.L. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
ELM reports consulting roles for Lilly, AstraZeneca, and Novartis. EAM reports compensated service on scientific advisory boards for AstraZeneca, BioNTech, Exact sciences (formerly Genomic Health), Merck, Moderna, Roche/Genentech; uncompensated service on steering committees for Bristol Myers Squibb, Lilly, and Roche/Genentech; speakers honoraria and travel support from Merck Sharp & Dohme; and institutional research support from Roche/Genentech (via SU2C grant) and Gilead. She receives research funding from Susan Komen for the Cure for which she serves as a Scientific Advisor. She also reports uncompensated participation as a member of the American Society of Clinical Oncology Board of Directors. TAK reports speaker honoraria and compensated service on scientific Advisory Board of Exact Sciences. She is also a Senior Associate Editor for Breast Cancer Research and Treatment. MCM reports consulting for Exact Sciences, Pfizer, Roche, Astra Zeneca, Lilly, Novartis, and Abbott; and research support from Novartis and Pfizer. LAN reports research support from Genentech. The remaining authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Brigham and Women’s Hospital.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Previous presentation:
This work was presented as an oral presentation at the American College of Surgeons Clinical Congress 2023, Boston, MA.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leonard, S., Jones, A.N., Newman, L. et al. Racial disparities in outcomes of patients with stage I-III triple-negative breast cancer after adjuvant chemotherapy: a post-hoc analysis of the E5103 randomized trial. Breast Cancer Res Treat 206, 185–193 (2024). https://doi.org/10.1007/s10549-024-07308-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-024-07308-8