Abstract
Purpose
We aimed to compare the initial and salvage brain-directed treatment and overall survival (OS) between patients with 1–4 brain metastases (BMs) and those with 5–10 from breast cancer (BC). We also organized a decision tree to select the initial whole-brain radiotherapy (WBRT) for these patients.
Methods
Between 2008 and 2014, 471 patients were diagnosed with 1–10 BMs. They were divided into two groups based on the number of BM: 1–4 BMs (n = 337) and 5–10 BMs (n = 134). Median follow-up duration was 14.0 months.
Results
Stereotactic radiosurgery (SRS)/fractionated stereotactic radiotherapy (FSRT) was the most common treatment modality (n = 120, 36%) in the 1–4 BMs group. In contrast, 80% (n = 107) of patients with 5–10 BMs were treated with WBRT. The median OS of the entire cohort, 1–4 BMs, and 5–10 BMs was 18.0, 20.9, and 13.9 months, respectively. In the multivariate analysis, the number of BM and WBRT were not associated with OS, whereas triple-negative BC and extracranial metastasis decreased OS. Physicians determined the initial WBRT based on four variables in the following order: number and location of BM, primary tumor control, and performance status. Salvage brain-directed treatment (n = 184), mainly SRS/FSRT (n = 109, 59%), prolonged OS by a median of 14.3 months.
Conclusion
The initial brain-directed treatment differed notably according to the number of BM, which was chosen based on four clinical factors. In patients with ≤ 10 BMs, the number of BM and WBRT did not affect OS. The major salvage brain-directed treatment modality was SRS/FSRT and increased OS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastasis (BM) has an extremely poor prognosis, and historically, only a few treatment options, such as whole-brain radiotherapy (WBRT), have been available [1, 2]. WBRT, as well as BM itself, could result in severe neurological dysfunction, decreasing patients’ quality of life (QoL) [2, 3]. However, multiple randomized controlled trials have demonstrated that patients with a limited number of BM can be treated with single-fraction stereotactic radiosurgery (SRS) alone without compromising overall survival (OS) and neurocognitive decline [4,5,6,7]. Based on these results, the concept of a limited number of BM, generally up to four BMs, was established [8].
The American Society for Radiation Oncology clinical practice guidelines, very recently published, strongly recommend, with high quality of evidence, SRS for intact 1–4 BMs patients with good performance status [9]. We previously reported that the proportion of patients who underwent SRS or fractionated stereotactic radiotherapy (FSRT) increased from 2005 to 2014 in Korea [10]. This trend accelerated in 2008 after changing the reimbursement coverage for SRS [10].
Considering the study period when representative clinical trials for patients with limited BM were conducted, a new definition of limited BM was required because novel systemic therapies were developed and improved intracranial tumor control [8]. The above guidelines also point out that there are a lack of evidence for the use of SRS in 5 or more BMs while suggesting SRS/FSRT as a treatment modality for 5–10 BMs patients [9].
In this study, we used large retrospective cohort data on BM from breast cancer (BC), in which most high-volume institutions in Korea were involved, to explore the possibility of extending the definition of limited BM to 10. Brain-directed treatment and OS were compared between patients with 1–4 and 5–10 BMs. Furthermore, we investigated the criteria for selecting WBRT for these patients.
Materials and methods
Study population
The Korean Radiation Oncology Group 16–12 study was a multicenter, retrospective cohort study conducted at 17 institutions in Korea. We reviewed the medical records of 730 patients with newly diagnosed BM from BC. In addition to the previously described inclusion and exclusion criteria [11], we narrowed down patients with 1–10 BMs since 2008, considering it was April 2007, the medical expenses for SRS were covered by the Korea Health Insurance Review and Assessment Service. Finally, 471 patients were included: 337 patients with 1–4 BMs and 134 with 5–10 BMs. Patient follow-up was updated only when possible. The median follow-up duration from the diagnosis of BM was 14.0 months (interquartile range, 6.3–26.5).
Tumor subtypes were classified into four categories based on immunohistochemical staining results: luminal A [hormone (estrogen and/or progesterone) receptor positive and human epidermal growth factor receptor 2 (HER2) negative], luminal B (hormone receptor positive and HER2 positive), HER2 (only HER2 positive), and triple negative (all negative).
The initial and subsequent brain-directed treatments are summarized in Table 1 and Fig. 1, respectively. The main treatment schemes for brain RT have been previously described [11,12,13]. The choice of treatment modality and prescription dose of RT was determined by each institution’s policy and attending physicians. Despite variations in the prescribed isodose lines for SRS across institutions, 13–25 Gy at the 50% isodose line was commonly used. FSRT and WBRT were administered at median total doses of 33 Gy in three fractions and 30 Gy in 10 fractions, respectively. In salvage settings, an SRS of 6–25 Gy was delivered at the 50% isodose line, and the median total dose of FSRT was 28 Gy in seven fractions. Patients received salvage WBRT at a median dose of 25 Gy in 10 fractions.
Statistical analysis
For baseline comparisons, the independent Student’s t test was used for continuous variables, and the chi-square or Fisher’s exact test was used for categorical variables. OS was estimated using the Kaplan–Meier method, and the log-rank test was used to compare groups. Cox proportional hazard models were used to identify prognostic factors affecting OS and describe hazard ratios (HRs) with 95% confidence intervals (CIs) using a backward elimination method.
The R rpart package was used to determine the WBRT. Variables identified in the univariate analysis were included in the analysis. The following conditions were used to generate the decision tree: at least 40 patients for a split to be attempted, a p-value of < 0.010 by the log-rank test, and tenfold cross-validation.
Two-sided tests showing a p-value < 0.050 were considered statistically significant. Analyses were performed using R version 4.1.2 (https://www.r-project.org/). Illustration was created using BioRender (https://app.biorender.com/).
Results
Patients with 5–10 BMs were mainly treated with WBRT
The baseline characteristics of the study population are presented in Table 1. Patients with 5–10 BMs had slightly longer intervals between BC and BM without statistical significance. However, a higher number of brain lesions was associated with a poor performance status (p = 0.064), extracranial metastasis (p = 0.006), and BMs in both tentorial regions (p < 0.001). Compared to patients with 1–4 BMs mostly managed with SRS/FSRT alone (35.6%), WBRT accounted for 79.9% of the initial brain-directed treatment for patients with 5–10 BMs, followed by SRS/FSRT (only 9.0%). Salvage brain-directed treatment was performed more frequently in patients with 1–4 BM than in those with 5–10 BMs (44.2% vs. 26.1%, p < 0.001).
In patients with ≤ 10 BMs, no correlation was found between BM number/WBRT and OS
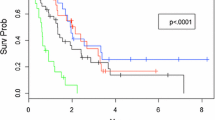
The median OS was 18.0 months (95% CI 15.6–21.5) in the entire cohort (Fig. 2a). According to the number of BM, a longer median OS was observed in the 1–4 BMs group than in the 5–10 BMs (20.9 months, 95% CI 17.6–25.7 vs. 13.9, 95% CI 10.9–17.6, p < 0.001, Fig. 2b). However, in the multivariate analysis (Table 2), the number of BM and initial WBRT did not decrease OS (p = 0.081 and 0.121, respectively). The prognostic factors for OS were triple negative BC (HR 1.632, 95% CI 1.268–2.100, p < 0.001), extracranial metastasis (HR 2.481, 95% CI 1.749–3.250, p < 0.001), and salvage brain-directed treatment (HR 0.559, 95% CI 0.439–0.711, p < 0.001).
The number of BM was the first node to select WBRT
Although there was no statistical difference in OS between patients with and without WBRT in our study, the initial WBRT was chosen according to Fig. 3. The decision tree consisted of four variables: the number and location of BM, control of primary BC, and performance status. First, physicians selected WBRT for patients with 5–10 BMs. Subsequently, for 1–4 BMs patients, the location of the BM, primary BC control, and performance status were assessed.
In addition, we compared whether there was a difference in OS between WBRT (+) and WBRT (-) in each subgroup classified by Fig. 3. Except for the second subgroup (patients with 1–4 BMs in both tentorial areas), OS in patients with WBRT was not different from that without WBRT (data are not shown). The WBRT selection algorithm showed that most physicians chose WBRT when patients had 1–4 BMs which were identified in both the supra- and infra-tentoria. But OS was better in WBRT (−), compared to WBRT (+), with a median OS of 25.4 and 11.9 months, respectively (p = 0.019). The aforementioned risk factors were evenly distributed among these patients. However, the rate of salvage brain-directed treatment was marginally higher in the initial WBRT (−) group (51.6% vs. 28.8%, p = 0.057).
Salvage treatment after initial brain-directed treatment prolonged OS
Salvage brain-directed treatment varied according to the number of BM and initial brain-directed treatment (Fig. 1). Regarding 1–4 BMs, salvage was frequently performed in patients who underwent SRS/FSRT alone (58.4%) or surgical resection (56.0%). SRS/FSRT was predominantly performed as a salvage treatment. In patients with 5–10 BMs, the salvage rate was lower than in those with 1–4 BMs. SRS/FSRT is the most commonly used salvage therapy.
The median OS after BM diagnosis of patients treated with salvage or not was 27.2 months (95% CI 23.0–34.0) and 12.9 (95% CI 10.9–15.5), respectively (p < 0.001, Fig. 4a). Salvage brain-directed treatment resulted in the median OS of 16.2 months (95% CI 11.9–22.0) after it (Fig. 4b). In these patients, the administration of initial WBRT did not affect the OS after salvage brain-directed treatment (p = 0.280).
Discussion
Our findings demonstrated that the OS of patients with 1–10 BMs depended not on the number of BM and initial use of WBRT but on triple-negative BC, extracranial metastasis, and salvage brain-directed treatment. For these patients, radiation oncologists did not consider WBRT as an initial brain-directed treatment when all the following criteria were met: (1) the number of BMs was less than five, (2) BM occupied only the supra- or infra-tentorium, (3) primary BC was well controlled, and (4) patients had a reasonable performance status. The present study also highlights the clinical importance of salvage brain-directed treatment for recurrent BM in terms of OS.
Patients with 5–10 BMs had poorer OS than those with 1–4 BMs in univariate analysis. Physicians tended to prefer WBRT when patients had 5–10 BMs. However, after adjusting for other clinical factors, the number of BM (1–4 BMs vs. 5–10 BMs) and WBRT were not associated with OS. Among the prognostic factors identified in the multivariate analysis, extracranial metastasis was more common in patients with 5–10 BMs, and the salvage rate was lower than that in those with 1–4 BMs. That is, the decreased OS in patients with 5–10 BMs could be explained by higher extracranial metastases and lower salvage treatment.
Japanese Leksell Gamma Knife Society (JLGK) 0901 was a prospective observational study recruiting patients with 1–10 BMs treated with SRS: the largest tumor < 10 mL, the longest diameter < 3 cm, and total cumulative volume ≤ 15 mL [14]. Non-inferiority of SRS to 5–10 BMs was observed compared to that of 2–4 BMs [14]. In this study, patients with BM were enrolled, irrespective of extracerebral disease control [14]. Although controlled extracerebral disease significantly favored longer survival (p = 0.001), the proportion of controlled/uncontrolled extracerebral disease was similar among the groups (1, 2–4, and 5–10 BMs) [14]. This might be one of the reasons why there was no difference in OS between 2–4 and 5–10 BMs (p = 0.94) [14]. Furthermore, the post-SRS cumulative rates of repeat SRS and WBRT did not differ between the 2–4 and 5–10 BMs [14]. These findings support our findings and emphasize the significance of extracranial tumor burden and salvage brain-directed treatment in these patients.
WBRT combined with SRS/FSRT can eradicate intracranial micrometastases that are not targeted by SRS/FSRT [15]. In a previous study, we demonstrated that WBRT could lower new BM development (i.e., distant intracranial failure), and BMs of more than four significantly increased the risk of new BM [12]. A meta-analysis showed better distant brain tumor control when WBRT plus SRS was administered compared with SRS alone [16]. In contrast, WBRT can prevent distant intracranial failure for only a maximum of 6 months [5]. Considering that the median OS of all patients was 18.0 months, patients who received WBRT eventually developed new BM. In contrast to WBRT, which showed no advantages in patients with 1–10 BMs, salvage treatment after initial brain-directed treatment had an OS benefit, with an OS improvement of 14.3 months. In the subgroup analysis of patients with 1–4 BMs in both tentoria, brain-directed treatment without WBRT resulted in better OS. This may be due to the higher salvage rate in the WBRT (−) group. This study revealed that salvage brain-directed treatment had a significant effect on OS. Because most salvage method was SRS/FSRT, additional SRS/FSRT may be possible for subsequent brain recurrences in previously untreated regions. Therefore, clinicians should be encouraged to offer SRS/FSRT to patients with 1–10 BMs and to defer WBRT as late as possible. This is also important in terms of the QoL of patients with a relatively small intracranial tumor burden.
As SRS/FSRT has become predominant, the concept of limited BM needs to be revised to suit the present era. The presence or control of extracranial metastasis is important in 1–10 BMs patients, rather than the number of BM, in our analysis. With the tremendous evolution of systemic treatment agents, the control of extracranial diseases has increased remarkably [2]. In addition, several innovative molecular-targeted therapies can penetrate the blood–brain barrier and achieve successful intracranial control, overcoming the drawbacks of SRS/FSRT, which could leave microscopic tumors untreated [2, 17, 18]. Although there should be a balance between concurrent systemic agents with SRS/FSRT and potential toxicities, novel systemic therapy options could expand the appropriate candidates for SRS/FSRT beyond the four BMs [8, 18].
However, there are inevitable limitations to be considered when interpreting our findings. First, the inherent flaws of this retrospective study, including selection bias, should be recognized. Despite a considerable database of patients with BM from BC, only a small number of patients were analyzed, and most patients in the 5–10 BMs group were treated with WBRT rather than SRS/FSRT, which might limit our proposal. In our analysis, other intracranial tumor burdens, such as the diameter or volume of the BM, which are also important factors associated with OS, were not included. However, in the JLGK0901, for 1–10 BMs that met the prespecified diameter and volume criteria of BM, the largest diameter (< 1.6 cm vs. ≥ 1.6 cm, p = 0.92) and cumulative tumor volume (< 1.9 mL vs. ≥ 1.9 mL, p = 0.24) were not associated with OS [14]. Considering the results of the JLGK0901 study [14], we assumed that these factors might modestly affect OS. Finally, external validation is required.
Conclusionally, when considering only the number, BMs fewer or equal to 10 did not affect the OS in our study population. Therefore, the number of BM should not be the highest priority in selecting WBRT, and selection should be made after a comprehensive deliberation of other factors. In addition, since no advantage of OS from WBRT was shown and the benefit of salvage treatment was clear, SRS/FSRT should be actively allowed as the first brain-directed therapy. It could maintain the patient’s QoL by preventing neurocognitive problems, while leaving the possibility of future salvage options. Eventually, we cautiously propose that BMs of up to 10 should be defined as limited BMs.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Jeon W, Jang BS, Jeon SH et al (2018) Analysis of survival outcomes based on molecular subtypes in breast cancer brain metastases: a single institutional cohort. Breast J 24:920–926. https://doi.org/10.1111/tbj.13111
Kim JS, Kim IA (2020) Evolving treatment strategies of brain metastases from breast cancer: current status and future direction. Ther Adv Med Oncol 12:175883592093611. https://doi.org/10.1177/1758835920936117
Quigley MR, Fukui O, Chew B et al (2013) The shifting landscape of metastatic breast cancer to the CNS. Neurosurg Rev 36:377–382. https://doi.org/10.1007/s10143-012-0446-6
Chang EL, Wefel JS, Hess KR et al (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037–1044. https://doi.org/10.1016/S1470-2045(09)70263-3
Aoyama H, Shirato H, Tago M et al (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. J Am Med Assoc 295:2483–2491. https://doi.org/10.1001/jama.295.21.2483
Kocher M, Soffietti R, Abacioglu U et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol 29:134–141. https://doi.org/10.1200/JCO.2010.30.1655
Brown PD, Jaeckle K, Ballman KV et al (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases a randomized clinical trial. JAMA - J Am Med Assoc 316:401–409. https://doi.org/10.1001/jama.2016.9839
Chaung KV, Sloan AE, Choi S (2021) Limited brain metastases: a narrative review. Ann Palliat Med 10:6016–6027. https://doi.org/10.21037/apm-21-363
Gondi V, Bauman G, Bradfield L et al (2022) Radiation therapy for brain metastases: an ASTRO clinical practice guideline. Pract Radiat Oncol 12:265–282. https://doi.org/10.1016/j.prro.2022.02.003
Kim JS, Kim K, Jung W et al (2022) The pattern of care for brain metastasis from breast cancer over the past 10 years in Korea: a multicenter retrospective study (KROG 16–12). Cancer Res Treat 54:1121–1129. https://doi.org/10.4143/crt.2021.1083
Kim JS, Kim K, Jung W et al (2020) Survival outcomes of breast cancer patients with brain metastases: a multicenter retrospective study in Korea (KROG 16–12). The Breast 49:41–47. https://doi.org/10.1016/j.breast.2019.10.007
Kim JS, Kim K, Jung W et al (2021) New brain metastases after whole-brain radiotherapy of initial brain metastases in breast cancer patients: the significance of molecular subtypes (KROG 16–12). Breast Cancer Res Treat 186:453–462. https://doi.org/10.1007/s10549-020-06043-0
Kim JS, Kim K, Jung W et al (2021) Novel prognostic classification predicts overall survival of patients receiving salvage whole-brain radiotherapy for recurrent brain metastasis from breast cancer: a recursive partitioning analysis (KROG 16–12). The Breast 60:272–278. https://doi.org/10.1016/j.breast.2021.11.005
Yamamoto M, Serizawa T, Shuto T et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15:387–395. https://doi.org/10.1016/S1470-2045(14)70061-0
Rogers S, Baumert B, Blanck O et al (2022) Stereotactic radiosurgery and radiotherapy for resected brain metastases: current pattern of care in the radiosurgery and stereotactic radiotherapy working group of the German association for radiation oncology (DEGRO). Strahlentherapie und Onkol 198:919–925. https://doi.org/10.1007/s00066-022-01991-6
Khan M, Lin J, Liao G et al (2017) Comparison of WBRT alone, SRS alone, and their combination in the treatment of one or more brain metastases: review and meta-analysis. Tumor Biol 39:101042831770290. https://doi.org/10.1177/1010428317702903
Yan H, Li X, Peng Y et al (2020) Apatinib and fractionated stereotactic radiotherapy for the treatment of limited brain metastases from primary lung mucoepidermoid carcinoma. Medicine (Baltimore) 99:e22925. https://doi.org/10.1097/MD.0000000000022925
Hendriks LEL, Schoenmaekers J, Zindler JD et al (2015) Safety of cranial radiotherapy concurrent with tyrosine kinase inhibitors in non-small cell lung cancer patients: a systematic review. Cancer Treat Rev 41:634–645. https://doi.org/10.1016/j.ctrv.2015.05.005
Funding
This work was supported by grants from Korean Ministry of Science and Information & Communication Technology (NRF#2023R1A2C3003782) to In Ah Kim.
Author information
Authors and Affiliations
Contributions
JSK contributed to formal analysis, methodology, visualization, writing of the original draft, and writing, reviewing, and editing of the manuscript. KK contributed to formal analysis, methodology, supervision, writing of the original draft, and writing, reviewing, and editing of the manuscript. WJ, KHS, SAI, HJK, YBK, JSC, JHK, DHC, YHP, DYK, THK, BOC, SWL, SK, JK, KMK, WKC, KSK, WSY, JHK, JC, and YKO contributed to investigation and writing, reviewing, and editing of the manuscript. IAK contributed to conceptualization, supervision, and writing, reviewing, and editing of the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The Institutional Review Board of each institution approved this study. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Consent to participate
The requirement for informed consent was waived because of the retrospective design.
Consent to publish
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jae Sik Kim currently working at the Department of Radiation Oncology in Soonchunhyang University Seoul Hospital.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, J.S., Kim, K., Jung, W. et al. Comparison of initial and sequential salvage brain-directed treatment in patients with 1–4 vs. 5–10 brain metastases from breast cancer (KROG 16–12). Breast Cancer Res Treat 200, 37–45 (2023). https://doi.org/10.1007/s10549-023-06936-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06936-w