Abstract
Purpose
Tumor-infiltrating lymphocytes (TILs) have been positively correlated with response to systemic therapy for triple-negative and HER2 + subtypes and improved clinical outcomes in early breast cancer (BC). Less is known about TILs in metastatic sites, particularly brain metastases (BM), where unique immune regulation governs stromal composition. Reactive glial cells actively participate in cytokine-mediated T cell stimulation. The impact of prior medical therapy (chemotherapy, endocrine, and HER2-targeted therapy) on the presence of TILs and gliosis in human breast cancer brain metastases (BCBM) has not been previously reported.
Methods
We examined prior treatment data for 133 patients who underwent craniotomy for resection of BMs from the electronic medical record. The primary endpoint was overall survival (OS) from the time of BM diagnosis. We examined the relationship between prior systemic therapy exposure and the histologic features of gliosis, necrosis, hemorrhage, and lymphocyte infiltration (LI) in BCBMs resected at subsequent craniotomy in univariate analyses.
Results
Complete treatment data were available for 123 patients. BCBM LI was identified in 35 of 116 (30%) patients who had received prior systemic treatment versus 5 of 7 (71.4%) who had not {significant by Fisher’s exact test p = 0.045}. There were no statistically significant relationships between prior systemic therapy and the three other histologic variables examined.
Conclusions
This observation suggests that systemic therapy may interfere with the immune response to BCBMs and cause exhaustion of anti-tumor immunity. This motivates clinical investigation of strategies to enhance LI for therapeutic benefit to improve outcomes for patients with BCBMs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most common cancer diagnosed in women and represents 14.8% of all new cancer cases in the United States of America (USA). Approximately 1 in 8 women (13%) will develop invasive BC during her lifetime. BC is the 4th most common cause of cancer death with an estimation of 43600 deaths in the USA in 2021 [1]. In the USA, BC is the 3rd most likely cancer to metastasize to the brain after lung cancer and melanoma. Approximately 10–30% of patients with BC will develop brain metastases (BM) during the course of their disease [2]. This can lead to a devastating effect on independence and quality of life and is often the limiting factor in survival.
The incidence of BM and overall survival (OS) is influenced by BC subtype. Patients with triple-negative breast cancer (TNBC) and human epidermal growth factor receptor 2 (HER2) overexpression have a higher risk of developing BM compared to luminal-HER2 negative disease [3].Treatment options for breast cancer brain metastases (BCBM) can be divided into systemic and local options. Systemic options include chemotherapy, endocrine therapy, and HER2 targeting agents. Local therapy options include surgery, stereotactic radiosurgery (SRS), and whole brain radiation therapy (WBRT).
Increased tumor-infiltrating lymphocytes (TILs) in BC predicted response to neoadjuvant therapy and showed a survival benefit in HER2+ and TNBC [4].This is thought to be due to necrotic tumor death causing release of stress proteins which triggers immune recognition of the tumor and enhances anti-tumor immunity. However, less is known about TILs in metastatic sites, particularly in BM as the brain microenvironment has unique immune regulation which governs stromal composition. We sought to investigate the impact of prior systemic therapy (chemotherapy, endocrine and HER2-targeted therapy) on the presence of TILs and gliosis in human BCBM.
Materials and methods
We extracted clinicopathological and prior treatment data from Jan 1991 to Dec 2006 for 133 patients who underwent craniotomy in Memorial Sloan Kettering Cancer Center for resection of BMs from the electronic medical record. The degrees of gliosis, immune infiltrate, hemorrhage, and necrosis were identified and scored via hematoxylin and eosin (H and E) staining (0−3+) on 5-µM thick tissue section slides from all craniotomy samples.
Criteria for scoring were as previously described by Hamilton et al. Immune infiltrate was quantitated by the presence of mononuclear cells around blood vessels and/or within the tumor parenchyma. Low infiltrate was defined as 0–2 perivascular infiltrates and high infiltrate was defined as >2 perivascular and/or any infiltrates within the tumor parenchyma. Tumor hemorrhage was defined by the presence of fresh hemorrhage, hematoidin-laden macrophages, organized blood clot, and ruptured vessels or hemorrhage adjacent to necrotic areas. Hemorrhage was quantitated as low with 0–2 foci of hematoidin, fresh blood, or organized clot; high with blood occupying >1/3 of the specimen. Necrosis was estimated as a percentage of necrotic tumor while gliosis was defined as the presence of reactive astrocytes only near the tumor [5].
We examined the relationship between prior systemic therapy exposure and the histologic features of gliosis, lymphocyte infiltration (LI), hemorrhage, and necrosis in BCBMs resected at subsequent craniotomy in univariate analyses.
For statistical analysis, all 4 biomarkers, gliosis, immune infiltrate, hemorrhage, and necrosis, were classified as absent or present, when present the extent was scored (0 vs. 1–3+). Necrosis was evaluated by highest degree vs. other (3+ vs. 1–2+). Figure 1 Differences in the biomarkers (present vs. absent, or highest degree vs. other) by the timing of pre-craniotomy systemic treatment were evaluated using Fisher's exact tests. Fisher’s exact test was chosen because of the small sample size in the no prior systemic treatment group. An alternative test (i.e., the Chi-square test) is based on large sample normal approximation, which could have led to erroneous conclusion in our small sample case. The primary endpoint was overall survival (OS) from the time of BM diagnosis. Kaplan–Meier estimates were constructed for OS for all samples and by each biomarker status. Log-rank tests were used to test whether OS differs by each biomarker status and Cox regression models were used to assess the impact of covariates which included local BM therapy received and the Breast Graded Prognostic Assessment (GPA) score. All statistical analyses were conducted using R 4.1.2 software and R package ‘survival’ version 3.3.1 was used for survival analysis. The follow-up time was censored at patients’ last known contact date for those who are alive.
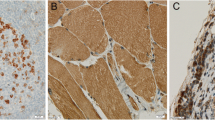
Description of histopathological biomarkers in H and Es of craniotomy samples. 1. Immune infiltrate, present (a) and absent (b). Arrows point to mononuclear cells within tumor tissue scored as immune infiltrate. 2. Gliosis, present (a) and absent (b). 3. Hemorrhage, present (a) and absent (b). 4. Necrosis, present (a) and absent (b)
Results
Complete treatment data were available for 123 patients. All 123 patients underwent a craniotomy for BM. Most of the patients had a craniotomy for a solitary or limited number of BM; however, a selected cohort of patients with multiple BM had a craniotomy for a target lesion to relieve obstructive symptoms. The median age of diagnosis with metastatic breast cancer (MBC) was 45 years (Interquartile range (IQR) 39-52). 53% of patients had a solitary BM, 38% had 2-9 BM, and 8% had 10 or more BM. 25% (n = 31) of patients had hormone receptor positive (HR+) HER2- BC and 40% (n = 49) had TNBC. 35% (n = 43) of the patients had HER2+ BC, including n = 15 (12%) with HR+/HER2+ BC and n = 28 (23%) with HR-/HER2+ BC. Subtype was determined according to the craniotomy specimen.
Twelve percent of patients (n = 14) presented with de novo MBC including 1 patient with BM at presentation, and 88% (n = 109) had relapsed MBC including 44 patients who had BM as first metastatic presentation. During the first presentation of MBC (both relapsed and de novo), 63% of patients had no BM, 22% had BM with extracranial disease, and 14% had BM only with no extracranial disease. Of the patients who had BM only at their first presentation with MBC the BM was HR+/HER2- in 4, 4 HER2+ in 4, and TNBC in 10. The highest incidence of presentation of BM at MBC diagnosis were in TNBC patients (47%). Median time to BM development from MBC diagnosis in this selected group of patients undergoing medically indicated craniotomy was 10 months (HR+/HER2-), 14 months (HER2+), and 2 months (TNBC).
Ninty four percent of patients received a form of systemic treatment pre-craniotomy either in the adjuvant or in the metastatic setting. 116 (94%) received chemotherapy, 49 (40%) received endocrine therapy, and 23 (19%) received HER2-targeted therapy (trastuzumab). A median of 1 line of systemic treatment was received pre-craniotomy (range 1–5). A small proportion of patients received radiotherapy (RT) pre-craniotomy with WBRT (7%) and SRS (3%) Table 1.
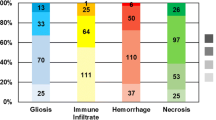
Across all tumor receptor subtypes, 51% had gliosis, 34% had LI, 76% had hemorrhage and 90% had necrosis. Of those who had necrosis it was scored as 3+ in 10% (n = 12) and 1–2+ in 80% (n = 94). In patients whose BM showed one or more of the aforementioned features, LI was most common in tumors from HER2+ patients (40%) while gliosis (35%), hemorrhage (39%), and necrosis (40%) were most common in TNBC patients.
Thirty five of of one hundred and sixteen patients (30%) who had received prior systemic treatment had BCBM LI while BCBM LI was observed in 5 of 7 patients (71.4%) who had not received systemic treatment {significant by Fisher’s exact test p = 0.045} Table 2. The odds ratio of LI was 0.17 when compared between patients who had received prior systemic treatment to those who did not. A small percentage of patients (n = 9, 7%) received WBRT prior to craniotomy, and there were no statistically significant relationships between prior WBRT and presence of the four biomarkers. Patients who received SRS prior to craniotomy were not included in the analysis as the location of initial SRS differed from the craniotomy site.
We note that 53% (n = 23) of patients with HER2+ BC received trastuzumab in the pre-craniotomy setting as this cohort is from a period where HER2-targeted therapy was not yet a standard of care. An additional 7 patients with HER2+ BC received trastuzumab in the post-craniotomy setting (70%). There were no statistically significant relationships between the timing of systemic treatment pre-craniotomy (adjuvant versus metastatic) and presence of the four biomarkers. Notably 97% (n = 123) of patients received dexamethasone within 7 days of craniotomy; there was no apparent difference in LI between those patients who had recent corticosteroid versus those who had not received it. All patients who did not receive systemic treatment prior to craniotomy received dexamethasone within 7 days of craniotomy.
OS from BM development according to subtype was 10 months (HR+HER2-), 32 months (HR+/HER2+), 26 months (HR-/HER2+), and 10 months (TNBC), respectively. The presence of gliosis was associated with an improvement in OS from BM development (p = 0.006) Figure 2. However, after multivariate analysis and adjusting for local BM treatment received and the Breast GPA score [6], the impact of gliosis on improved OS became borderline significant (p = 0.05). There were no statistically significant relationships between OS and LI, necrosis and hemorrhage Table 3. Due to a relatively modest sample size, we did not have enough power to test for statistical significance of the four biomarkers in each receptor subtype.
Discussion
The survival and growth of metastases depends on how tumor cells interact with the host organ microenvironment. The brain microenvironment is different from extracranial sites, where stromal composition is governed by unique immune regulation. Cancer immunoediting is a concept that the immune system shapes tumor immunogenicity and protects against tumor progression. It consists of 3 main phases, which are elimination, equilibrium, and escape. The elimination phase is a state of cancer immunosurveillance where innate and adaptive immune systems detect and eliminate cancer cells before they become clinically apparent. The equilibrium phase is where residual tumor variants which are not eliminated are kept in a dormant state by immunoediting and are prevented from tumor outgrowth. However, due to selective pressure, certain tumor cells enter the escape phase and learn to evade immunogenic destruction and become growing tumors [7].
In melanoma, a high immune infiltrate in BM was found to correlate with an improved OS [5].Improved survival was also seen in glioblastomas with intermediate to extensive lymphocytic infiltration [8].The presence of TILs in early BC is predictive of improved long-term outcomes. However, less is known about the changes in TIL distribution during MBC progression and whether there is a difference in TIL presence in extracranial and intracranial metastases. HER2+ and TNBC metastases were found to have a lower percentage of TILs compared to their primary tumors, which suggests that immune escape plays a role in tumor progression [9].
A focused examination of TILs, programmed cell death ligand 1 and 2 (PD-L1 and 2) expression, and glial fibrillary proteins in BCBM and their microenvironment found that PD-L1 expression on TILs had a favorable prognostic impact. This supports the beneficial effect of preexisting anti-tumor immunity [10].PD-L1 expression was found to be more common in primary HER2+ and TNBC cancers and was associated with increased TILs infiltration and a possible lower relapse rate [11]. Patients with TNBC who had high levels of TILs infiltration were also found to have an improved disease free survival [12]. A cohort of BM which included breast, melanoma, and lung cancers found that the presence of TILs and PD-L1 expression showed a negative correlation with BM size. This could suggest that the TILs could have initially controlled tumor size before becoming exhausted [13].
Microglia cells are an important component of the brain’s immune system and function as phagocytes and antigen presenting cells. When brain injury occurs, these cells are activated and infiltrate the site of injury to form a neuroinflammatory response [14].Their contribution to the progression of BM remains unclear, with both pro - and antitumorigenic roles being proposed. In an antitumorigenic role, microglia produce plasminogen activators upon encountering metastatic cells which cause the elimination of cancer cells that cross the blood–brain barrier (BBB) [15].Mouse studies using melanoma, lung, and colon cancer cell lines found that astroglia stimulated to produce nitric oxide by the addition of cytokines inhibited the growth of BM [16]. Sambade et al noted a superior prognosis from BM diagnosis in TNBC with gliosis, while LI was associated with an added benefit in HER2+ patients [17].This is supported by our findings of patients having an improved time from BM survival if gliosis was present. We did not note any statistically significant relationship between OS and LI which is likely due to our modest cohort.
In a protumorigenic role, metastatic tumor cells could potentially hijack glial cells to secrete factors that alter the brain microenvironment to promote tumor growth [18].Murine models suggest that melanoma cells can reprogram normal glial cells to express serine protease inhibitors (SERPINS), thus promoting brain metastatic growth rather than inhibiting it [19]. Mouse studies of BC cell lines found that tumor cells in the brain highly expressed interleukin-1β (IL-1β) which then activated surrounding astrocytes. This in turn stimulated Notch signaling and promoted BCBM growth [20].
Chemoradiation has been noted to increase TIL response in the neoadjuvant setting of rectal cancer which leads to improved outcomes. This is thought to be due to necrotic tumor death causing release of stress proteins which triggers immune recognition of the tumor and enhances anti-tumor immunity [21].In the early BC setting, there has been differing opinions on the impact of TILs levels with RT and OS. In the DBCG82bc trial, patients with high TILs were found to have improved OS post adjuvant RT, and this was particularly marked in ER- tumors (8% OS improvement for low TILs vs. 22% for high TILs, P = 0.028) [22]. However, the SweBCT91RT trial found that adjuvant RT in early BC was significantly beneficial in the low TILs group (hazard ratio 0.37; 95% confidence interval, 0.24 to 0.58; p < .001) but not in the high TILs group. The test for interaction between RT and TILs was not statistically significant (p = 0.317) [23]. The difference in the TILs cutoff threshold used between these two studies (10% vs 50%) may contribute to the difference in findings.
Our findings of decreased BCBM LI in patients who received prior systemic therapy is hypothesis generating in suggesting that there may have been an exhaustion of anti-tumor immunity. Checkpoint inhibitors are a promising option of treatment that could help restore anti-tumor immunity. IMpassion130 showed a progression free survival advantage with the addition of atezolizumab in metastatic TNBC patients who expressed PD-L1. Patients with asymptomatic treated BM were permitted and made up 13% of the study cohort [24]. Similarly, Keynote-355 showed a progression free survival benefit with the addition of pembrolizumab to metastatic TNBC and enrolled patients with asymptomatic BM which made up of 6% of the study population [25]. Patients with BCBMs are traditionally underrepresented in immunotherapy trials, and results from upcoming trials that focus on patients with BCBM are eagerly awaited [26,27,28]. A recent trial with autologous neoantigen TILs and pembrolizumab in MBC patients shows promising objective responses and could pave the way for personalized immunotherapy treatment [29].
To our knowledge, there has been limited research reported on prior treatment impact on TILS in BM, and our study is one of the first to characterize this. A limitation of our study is that in our cohort, there was a relatively small number of patients who did not receive prior systemic therapy. There is a possible inherent bias that only fit patients who were estimated to have a reasonable post-operative survival were more likely to have been referred for surgery. TILs infiltration was not characterized beyond H and E staining. We do not have data regarding the size of the initial brain metastases, which might correlate with TIL activation. Mouse models have shown a positive correlation between the volume of astrocyte activation and tumor volume [30]. As our cohort derives from the 1990s, the array of systemic treatment available prior to craniotomy is narrower compared to the current modern-day complement. This particularly includes anti-HER2-targeted treatment and checkpoint inhibitors in the neoadjuvant and adjuvant setting.
Conclusion
Patients had improved survival from time of BM diagnosis if gliosis was present. We observed decreased BCBM LI in patients who received systemic therapy prior to craniotomy which suggests that systemic therapy may interfere with the immune response to BCBMs. This motivates clinical investigation of strategies to enhance LI for therapeutic benefit to improve the outcomes for patients with BCBMs.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to patient confidentiality but are available from the corresponding author on reasonable request.
Abbreviations
- BBB:
-
Blood–brain barrier
- BM:
-
Brain metastases
- BC:
-
Breast cancer
- BCBM:
-
Breast cancer brain metastases
- GPA:
-
Graded Prognostic Assessment
- H and E:
-
Hematoxylin and eosin
- HR:
-
Hormone receptor
- HER2:
-
Human epidermal growth factor receptor 2
- IL-1β:
-
Interleukin-1β
- IQR:
-
Interquartile range
- LI:
-
Lymphocyte infiltration
- MBC:
-
Metastatic breast cancer
- OS:
-
Overall survival
- PD-L1:
-
Programmed cell death ligand 1
- RT:
-
Radiotherapy
- SERPINS:
-
Serine protease inhibitors
- SRS:
-
Stereotactic radiosurgery
- TNBC:
-
Triple-negative breast cancer
- TILs:
-
Tumor-infiltrating lymphocytes
- USA:
-
United States of America
- WBRT:
-
Whole brain radiation therapy
References
Cancer of the Breast (Female) - Cancer Stat Facts SEER. [cited 2022 Aug 1]. Available from: https://seer.cancer.gov/statfacts/html/breast.html
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22:2865–2872. https://doi.org/10.1200/JCO.2004.12.149
Kennecke H, Yerushalmi R, Woods R, Cheang M, Voduc D, Speers CH, Nielsen TO, Gelmon K (2010) Metastatic Behavior of Breast Cancer Subtypes. JCO 28:3271–3277. https://doi.org/10.1200/JCO.2009.25.9820
Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI et al (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19:40–50. https://doi.org/10.1016/S1470-2045(17)30904-X
Hamilton R, Krauze M, Romkes M, Omolo B, Konstantinopoulos P et al (2013) Pathologic and gene expression features of metastatic melanomas to the brain (MBM). Cancer 119:2737–2746. https://doi.org/10.1002/cncr.28029
Sperduto PW, Mesko S, Li J, Cagney D, Aizer A et al (2020) Beyond an updated graded prognostic assessment (Breast GPA): a prognostic index and trends in treatment and survival in breast cancer brain metastases from 1985 to today. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2020.01.051
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331:1565–1570. https://doi.org/10.1126/science.1203486
Yang I, Tihan T, Han SJ, Wrensch MR, Wiencke J, Sughrue ME, Parsa AT (2010) CD8+ T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. J Clin Neurosci 17:1381–1385. https://doi.org/10.1016/j.jocn.2010.03.031
Ogiya R, Niikura N, Kumaki N, Bianchini G, Kitano S et al (2016) Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients. Cancer Sci 107:1730–1735. https://doi.org/10.1111/cas.13101
Duchnowska R, Pęksa R, Radecka B, Mandat T, Trojanowski T et al (2016) Immune response in breast cancer brain metastases and their microenvironment: the role of the PD-1/PD-L axis. Breast Cancer Res 18:43. https://doi.org/10.1186/s13058-016-0702-8
Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y et al (2016) PD-L1 (B7–H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol 47:52–63. https://doi.org/10.1016/j.humpath.2015.09.003
Zhu X, Zhang Q, Wang D, Liu C, Han B, Yang JM (2019) Expression of PD-L1 attenuates the positive impacts of high-level tumor-infiltrating lymphocytes on prognosis of triple-negative breast cancer. Cancer Biol Ther 20(8):1105–1112. https://doi.org/10.1080/15384047.2019.1595282
Harter PN, Bernatz S, Scholz A, Zeiner PS, Zinke J et al (2015) Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain metastases. Oncotarget 6:40836–40849. https://doi.org/10.18632/oncotarget.5696
Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL (2005) Role of microglia in the central nervous system’s immune response. Neurol Res 27:685–691. https://doi.org/10.1179/016164105X49463a
Wasilewski D, Priego N, Fustero-Torre C, Valiente M (2017) Reactive astrocytes in brain metastasis. Front Oncol 7:298. https://doi.org/10.3389/fonc.2017.00298
Samdani AF, Kuchner EB, Rhines L, Adamson DC, Lawson C, Tyler B, Brem H, Dawson VL, Dawson TM (2004) Astroglia induce cytotoxic effects on brain tumors via a nitric oxide-dependent pathway both in vitro and in vivo. Neurosurgery. https://doi.org/10.1227/01.neu.0000119576.76193.b8
Sambade MJ, Prince G, Deal AM, Trembath D, McKee M et al (2019) Examination and prognostic implications of the unique microenvironment of breast cancer brain metastases. Breast Cancer Res Treat 176:321–328. https://doi.org/10.1007/s10549-019-05211-1
Fitzgerald DP, Palmieri D, Hua E, Hargrave E, Steeg PS et al (2008) Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis 25:799–810. https://doi.org/10.1007/s10585-008-9193-z
Schwartz H, Blacher E, Amer M, Livneh N, Abramovitz L et al (2016) Incipient melanoma brain metastases instigate astrogliosis and neuroinflammation. Cancer Res 76:4359–4371. https://doi.org/10.1158/0008-5472.CAN-16-0485
Xing F, Kobayashi A, Okuda H, Watabe M, Pai SK et al (2013) Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. EMBO Mol Med 5:384–396. https://doi.org/10.1002/emmm.201201623
Teng F, Mu D, Meng X, Kong L, Zhu H, Liu S, Zhang J, Yu J (2015) Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am J Cancer Res 5:2064–2074
Tramm T, Vinter H, Vahl P, Özcan D, Alsner J, Overgaard J (2022) Tumor-infiltrating lymphocytes predict improved overall survival after post-mastectomy radiotherapy: a study of the randomized DBCG82bc cohort. Acta Oncol 61:153–162. https://doi.org/10.1080/0284186X.2021.1989629
Kovács A, Stenmark Tullberg A, Werner Rönnerman E, Holmberg E, Hartman L et al (2019) Effect of radiotherapy after breast-conserving surgery depending on the presence of tumor-infiltrating lymphocytes: a long-term follow-up of the SweBCG91RT randomized trial. J Clin Oncol 37:1179–1187. https://doi.org/10.1200/JCO.18.02157
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108–2121. https://doi.org/10.1056/NEJMoa1809615
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA et al (2020) Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396:1817–1828. https://doi.org/10.1016/S0140-6736(20)32531-9
Stereotactic Radiation and Nivolumab in the Management of Metastatic Breast Cancer Brain Metastases. [cited 2022 Aug 1]. Available from: https://clinicaltrials.gov/ct2/show/NCT03807765
Atezolizumab in Combination With Pertuzumab Plus High-dose Trastuzumab for the Treatment of Central Nervous System Metastases in Patients With Her2-positive Breast Cancer [cited 2022 Aug 1]. Available from: https://clinicaltrials.gov/ct2/show/NCT03449238
Pembrolizumab And Stereotactic Radiosurgery (SRS) Of Selected Brain Metastases In Breast Cancer Patients [cited 2022 Aug 1]. Available from: https://clinicaltrials.gov/ct2/show/NCT03417544
Zacharakis N, Huq LM, Seitter SJ, Kim SP, Gartner JJ et al (2022) Breast cancers are immunogenic: immunologic analyses and a phase ii pilot clinical trial using mutation-reactive autologous lymphocytes. J Clin Oncol. https://doi.org/10.1200/JCO.21.02170
O’Brien ER, Kersemans V, Tredwell M, Checa B, Serres S et al (2014) Glial activation in the early stages of brain metastasis: TSPO as a diagnostic biomarker. J Nucl Med 55(2):275–280. https://doi.org/10.2967/jnumed.113.127449
Acknowledgements
We would like to thank Dr Jedd Wolchok from Memorial Sloan Kettering Cancer Center, Immuno-Oncology Service, New York, USA, for his advice and comments.
Funding
This research received no external funding. Dr. Anders is a Translating Duke Health Scholar and is supported by the Translating Duke Health Initiative (TDHI).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by AB, SC, AD, MM, MS, and DT, Supervision was performed by CA, EB, and AS. Statistical analysis was performed by YC. The first draft of the manuscript was written by SC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
CKA discloses Research funding: PUMA, Lilly, Merck, Seattle Genetics, Nektar, Tesaro, G1-Therapeutics, ZION, Novartis, Pfizer, Elucida; Compensated consultant role: Genentech, Eisai, IPSEN, Seattle Genetics, Astra Zeneca, Novartis, Immunomedics, Elucida, Athenex; Royalties: UpToDate, Jones and Bartlett. The other authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical review and approval were waived for this study, as this was a retrospective observational study of data obtained for clinical purposes. This study was registered under Memorial Sloan Kettering IRB waiver #WA0125-06.
Informed consent
Patient consent was waived due to retrospective data being collected anonymously for a quality improvement process that aimed to improve patient care and outcomes through systematic review of care against explicit criteria and standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The abstract of this paper was presented at the 2018 ASCO Annual Meeting as a poster presentation with interim findings. The abstract was published in Journal of Clinical Oncology 2018 36:15_suppl, 2063-2063. DOI: 10.1200/JCO.2018.36.15_suppl.2063 Journal of Clinical Oncology 36, no. 15_suppl (May 20, 2018) 2063-2063.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chew Minmin, S., Bacotti, A., Chen, Y. et al. Impact of prior systemic therapy on lymphocytic infiltration in surgically resected breast cancer brain metastases. Breast Cancer Res Treat 199, 99–107 (2023). https://doi.org/10.1007/s10549-023-06908-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06908-0