Abstract
Purpose
Pathogenic variants (PVs) in BRCA1 and BRCA2 genes are essential biomarkers of an increased breast and ovarian cancer risk and tumor sensitivity to poly ADP ribose polymerase inhibitors. In Russia, eight PVs were thought to be the most common, among which BRCA1 c.5266dup is the most frequently identified one.
Methods
We show the distribution of BRCA1/2 PVs identified with quantitative PCR and targeted next-generation sequencing in 1399 ovarian cancer patients recruited into the study from 72 Russian regions in 2015–2021.
Results
The most abundant PVs were c.5266dup (41.0%), c.4035del (7.0%), c.1961del (6.3%), c.181 T > G (5.2%), c.3756_3759del (1.8%), c.3700_3704del (1.5%), and c.68_69del (1.5%), all found in BRCA1 and known to be recurrent in Russia. Several other frequent PVs were identified: c.5152 + 1G > T (1.2%), c.1687C > T (1.0%), c.4689C > G (0.9%), c.1510del (0.6%), c.2285_2286del (0.6%) in the BRCA1 gene; and c.5286 T > G (1.2%), c.2808_2811del (0.8%), c.3847_3848del (0.8%), c.658_659del (0.7%), c.7879A > T (0.6%), in the BRCA2 gene. For the most common PV in the BRCA2 gene c.5286 T > G, we suggested that it arose about 700 years ago and is a new founder mutation.
Conclusion
This study extends our knowledge about the BRCA1 and BRCA2 pathogenic variants variability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
BRCA1 and BRCA2 are the genes that often contain a germline pathogenic variant in ovarian cancer patients [1]. Their influence on genome maintenance is associated with their essential role in the repair of double-strand breaks. This link has led to the development of new targeted therapy with poly ADP ribose polymerase inhibitors that show more effective and less toxic treatment than standard chemotherapy [2]. Several such drugs have been approved for use by the U.S. Food and Drug Administration (olaparib, rucaparib, and niraparib), and some new are in the late stage of clinical development [3]. The main indication for the use of such drugs is the presence of pathogenic variants in the BRCA1 or BRCA2 gene. Therefore, their occurrence in a population is necessary for effective organization of mutation carrier screenings.
Today, more than 6500 pathogenic and likely pathogenic variants (PVs) are known for the BRCA1 and BRCA2 genes according to the ClinVar database, and this number increases every year. The occurrence of the PVs varies between different populations significantly, including the prevalence of the most frequent variants. Depending on the population, ten most common PVs can compose from 33 to 89% of all carriers [4,5,6,7,8,9]. For populations with a high contribution of founder mutations, population prescreening with simple methods like qPCR can be applied as suggested for the USA Ashkenazi patients over many years [10].
The BRCA1/2 pathogenic variant frequency in Russia strongly deviates to several recurrent mutations (c.5266dup, c.4035del, and c.68_69del in the BRCA1 and c.5946del in the BRCA2) [11]. Recently a study with PCR, Sanger, and next-generation sequencing showed that whole-coding BRCA1/2 gene analysis in Russia could increase the number of PV carriers identified twice [11]. However, the whole-coding data were obtained only for 785 patients with breast or ovarian cancer, among which only 117 were PV carriers which is not enough for an unbiased mutation frequency estimation. In the meantime more than 13,000 and 73,000 new cases of ovarian and breast cancers are registered in Russia every year, respectively, and so far, there have been no studies based on NGS in which the proportion of ovarian or breast cancer cases with a mutation in the BRCA1/2 genes has been estimated because most of previous studies used qPCR tests for several hotspot PVs. Therefore, to determine the occurrence of already known highly recurrent PVs and to identify new PVs, we studied BRCA1 and BRCA2 genes with targeted next-generation sequencing (NGS) and quantitative PCR (qPCR) in 1399 ovarian cancer patients. To our knowledge, this is the largest study of whole-coding sequences of the BRCA1/2 genes for Russian populations.
Materials and methods
Subjects
One thousand three hundred ninety-nine unrelated and unselected for family history ovarian cancer patients with revealed germline BRCA1/2 pathogenic variant were retrospectively recruited by two main centers in Moscow and one in Novosibirsk in 2015–2021. Only citizens of Russia were involved in the study. The BRCA1/2 testing was carried out as a routine diagnostic at the request of the patient’s attending physician in order to choose treatment tactics and assess the risk of hereditary syndrome. In Russia, the procedure is regulated by the standards of medical care of the Ministry of Health of the Russian Federation, according to which (as of October 12, 2022) all patients with high-grade serous or endometrioid carcinomas are recommended to undergo molecular genetic testing of BRCA1/2 genes in DNA from blood leukocytes, oral mucosa and/or biopsy or surgical material. In most cases, DNA from blood leukocytes is not tested for pathogenic variants detected in tumor material, and therefore it is impossible to accurately determine the pathogenic variant status (germline or somatic). Therefore, all samples from the tumor tissue were not included into the study. The results do not include any further data on the patients’ response to treatment or outcome.
The median age of the patients recruited at the time of testing BRCA1/2 genes was 53 (Q1–Q3 was 46–60) among 265 patients for which the data were available. All ovarian cancer cases were high-grade serous adenocarcinoma with unknown personal history of breast cancer. Among 1056 patients for which the region was known, patients were from 72 different regions. The highest numbers were from Moscow (114), Primorsky Krai (88), Novosibirsk Oblast (76), and the Moscow region (71). Due to the difficulties with collecting data on the ethnicity of patients, we took into account only their place of residence, which correlates with ethnicity in Russia. DNA from blood leukocytes was extracted using an in-house method comprising cell lysis using 10% sodium dodecyl sulfate (SDS) containing buffer, proteinase K treatment, protein extraction using phenol–chloroform, and isopropanol precipitation of the DNA.
BRCA1 and BRCA2 genes mutation screening
Most of highly recurrent PVs (771 patients with a PV, BRCA1: c.5266dup (5382insC), c.4035del (4154delA), c.1961del (2080delA), c.181 T > G (C61G), c.3756_3759del (3875delGTCT), c.3700_3704del (3819delGTAAA), c.68_69del (185delAG), BRCA2: c.5946del (6174delT)) were identified with quantitative polymerase chain reaction (qPCR) (Supplementary Table 1). For every patient without hotspot PV and also for 141 patients for which hotspot PV was initially identified with targeted NGS, the BRCA1 and BRCA2 coding sequences were studied using the in-house amplicon-based targeted NGS panel (564 patients), GeneRead QIAact BRCA 1/2 panel (Qiagen) (64 patients). Coding exons, splice-acceptor, and splice-donor sites were covered (transcripts NM_007294.3 and NM_000059.3). NGS libraries were sequenced with MiSeq and MiniSeq Illumina platforms (348 patients) or with Ion S5/Ion Chef System (ThermoFisher) (280 patients) (Fig. 1). The NGS data were analyzed with the BRCA-analyzer [12] or Torrent Suite software followed by ANNOVAR annotation [13]. Visual data analysis, manual filtering of sequencing artifacts, and sequence alignments were performed using the Integrative Genomics Viewer (IGV) [14]. HGVS nomenclature was verified with the VariantValidator tool [15]. All statistical analyses were performed using scipy Python package [16].
The scheme of recruiting patients into the study and BRCA1/2 pathogenic variant screening. All numbers reflect only patients positive for BRCA1 or BRCA2 PV. 51 patients for which PVs were identified with targeted NGS in Istra (Moscow Region) were negative in qPCR tests for the most recurrent PVs. In Novosibirsk, 297 patients were tested in the same way, meanwhile for 141 patients, PVs were identified only with targeted NGS. RCMG—Research centre for medical genetics; MCOH—Moscow city oncology hospital No 62 of the Moscow health department; ICBFM SB RAW—Institute of chemical biology and fundamental medicine, Siberian branch of the Russian academy of sciences
Mutation age estimates
To estimate the age of PV BRCA2 c.5286 T > G for which we suggested the founder effect and the number of DNA samples available for the analysis was acceptable, we designed a new amplicon-based NGS panel targeting single nucleotide polymorphisms (SNPs) flanking ± 5 Mb around the BRCA2 gene (28 SNPs with CEU population frequency ≥ 30%) with the NGS-PrimerPlex program [17] (Supplementary Table 2). SNPs were chosen based on the minor allele frequency and the allele linkage disequilibrium (LD) from the LDProxy tool (https://ldlink.nci.nih.gov/). The next SNP was selected so that the LD with the previous one would be less than 0.7. Primer sequences are in Supplementary Table 2. Phased genotypes were obtained using the 1000Genomes [18] data and the Beagle tool [19]. Finally, the mutation age was estimated with the Mutation dating online tool [20].
Results
Pathogenic and likely pathogenic variants with high frequency
For 1399 patients positive for a deleterious BRCA1/2 PV, the occurrences of the most frequent (≥ 7 samples) variants are presented in Table 1. The whole list of PVs identified is in Supplementary Table 3. For 1161 (83%) and 238 (17%) patients, the PV was in the BRCA1 or BRCA2 gene, respectively, corresponding to 245 unique PVs, 128 (52%) and 117 (48%) in the BRCA1 and BRCA2 genes, respectively. The number of variants in the ovarian cancer cluster region (OCCR) and the breast cancer cluster region (BCCR) [21] was 90 (36.7%) and 66 (26.9%) which corresponded to 424 (30.3%) and 758 (54.2%) participants, respectively.
Due to the founder effect known for several PVs detected (c.5266dup, c.4035del, c.1961del, c.181 T > G), we evaluated the variant occurrence ratios in different regions of Russia (Fig. 2). For most regions with at least 20 PV carriers, the c.5266dup variant occurred in 39–80%, except for the Khanty-Mansi Autonomous Okrug (the administrative center is Khanty-Mansiysk, only 18% of 34 carriers) that could be associated with indigenous peoples living in the region. The total occurrence of the four highly recurrent variants varied between 44 and 88% depending on the region with the lowest values for the Khanty-Mansi Autonomous Okrug and the Chelyabinsk oblast (the administrative center is Chelyabinsk) regions. Therefore, screening healthy population for hotspot PVs is less justified for these two regions.
The PV occurrence in different regions of Russia. The pie charts are shown in the regional administrative centers. For regions with more than 20 PV carriers identified, the pie chart radiuses reflect the number of PV carriers found, and administrative region capitals are written. The threshold was chosen based on the convenience of the map element locations. For the most of regions, BRCA1 c.5266dup is the most recurrent PV (red color)
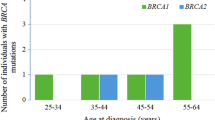
For 265 patients with known ages at time of testing BRCA1/2 genes, the number of cases younger than 50 was statistically significantly more frequent for patients with PV in the BRCA1 gene than for BRCA2 PV carriers (p-value = 0.0135 in the two-sided Fisher exact test) (Fig. 3). And this trend has persisted with the exclusion of c.5266dup variant carriers (p-value = 0.0425 in the two-sided Fisher exact test). These results correspond to the literature data about the earlier disease onset and a higher comulative risk of ovarian cancer in patients with BRCA1 PV than BRCA2 PV carriers [29].
For some of the PVs detected, the founder effect was suggested earlier (Table 1), and others were found in several cases for the first time. Therefore, we provided the literature review for all such variants, and for the BRCA2 c.5286 T > G, we estimated the mutation age.
New founder mutation BRCA2 c.5286 T > G
BRCA2 c.5286 T > G was found in 17 ovarian cancer patients from different Russian regions (Moscow, Novosibirsk, Irkutsk, Kirov, and Chelyabinsk Oblasts, Yakutiya, Primorsky Krai), and the variant common origin was suggested. To confirm it, we compared the BRCA2 gene SNPs identified by sequencing BRCA1/2 exons by a targeted NGS panel. For six patients, 12 flanking SNP phased alleles (from 32,888,483 to 32,931,875, hg19 human genome assembly) were the same. We developed a new small targeted NGS panel covering 28 SNPs around the BRCA2 gene to estimate the mutation age. For the moment of this study stage, the DNA samples were available only for six patients. The number of generations since the last common shared ancestor was determined as 34.8 (700 years, CI95:19.6–62.2) and 41.1 (820 years, CI95:19.6–62.2) for an independent and correlated genealogy, respectively. This mutation age is less than for the c.5266dup determined earlier (about 72 generations) [30], and this value needs to be re-estimated for the higher number of samples and variations.
Other BRCA2 highly recurrent PVs
Five BRCA2 PVs have earlier been determined as founder ones in Europe: c.2808_2811del (11 patients, found in Spain), c.3847_3848del (11 patients, Denmark and Norway), c.658_659del (10 patients, Lithuania), c.7879A > T (9 patients, Macedonia), c.5946del (also known as 6174delT, seven patients, Ashkenazi Jews, and Hungary) (Table 2).
BRCA1 highly recurrent PVs
Five BRCA1 PVs were identified in at least eight patients: c.5152 + 1G > T (17 patients), c.1687C > T (14 patients), c.4689C > G (13 patients), c.1510del (9 patients), and c.2285_2286del (8 patients). Due to the absence of the genotyping system, we couldn’t compare genotypes of the flanking SNPs for these BRCA1 PVs. However, we reviewed their occurrence in other studies. c.1687C > T is a known founder variant in Austria, Slovenia, and Sweden [22]; c.4689C > G was earlier identified in many patients in Germany, the USA, and Russia [24]. However, c.5152 + 1G > T was found only in two Russian patients [31, 32] and in several families in the worldwide study [33]; c.1510del was earlier found in some studies [34,35,36]; c.2285_2286del is a previously undescribed PV. These variants were observed more frequently than the BRCA2 c.5946del, thought to be highly reccurent in Russia.
Discussion
Here, we confirmed that BRCA1 c.5266dup was the most abundant germline pathogenic variant in Russian ovarian cancer patients accounting for up to 50% of all BRCA1/2 PV cases. However, its frequency is significantly lower than 90%, as it was thought before the start of NGS application when most pathogenic variants were identified with qPCR [37]. Its high occurrence in Russia can be explained by the spreading from Scandinavia or northern Russia about 1800 years ago, as suggested [30]. And now we can observe its high prevalence over other PVs in different Russian regions, from Kaliningrad in the west to Yuzhno-Sakhalinsk in the east. Therefore, screening some groups of Russian healthy populations for only this mutation could be considered to help in the early detection of patients with a high risk of breast and ovarian cancer. This variant also has a high frequency in different European countries with a rapid decrease in frequency from east to west and in many countries in North and South America, mainly in people with European ancestry [38]. Other recurrent PVs (c.4035del, c.181 T > G, c.1961del, c.68_69del, c.3756_3759del, and c.3700_3704del) also had a wide distribution in Russia without any region prevalence that is similar to results of previous studies in Poland, Ukraine, Latvia, Czech Republic, and Lithuania [33, 38, 40, 41]. Three of the most frequent PVs are the same as in Israel: c.68_69del, c.5266dup, c.181 T > G [34]. Similar results were obtained in a recent worldwide study, where 160 PV carriers from Russia participated [33]. The data showed that c.5266dup is the most abundant worldwide PV followed by c.68_69del and c.5946del, and a PV frequency was highly dependent on the geographic region that can be useful for further research on the history of PV spread. Some PVs identified here were observed in other countries: c.1687C > T (Austria, 14 participants in this study), c.4689C > G (Germany, 13 participants) c.4327C > T (Canada, 3 participants), c.5503C > T (Australia, 4 participants), and the common ancestral origin can be suggested. For example, c.4327C > T is a known founder pathogenic variant in the Quebec population [42] and was likely introduced from that population. Interestingly, 54.2% of ovarian cancer patients who participated in this study contained the PV in the BCCR versus 30.3% with a mutation in the OCCR, that do not contradict the hypothesis of the existence of the cancer-specific gene regions [21]. This result only shows that the occurrence of pathogenic variants in the BCCR is almost twice higher than in the OCCR.
To our knowledge, we identified 44 previously undescribed PVs in the BRCA1 and BRCA2 genes in 51 participants, which was 18% of the total number of different PVs detected in this study and 3.6% of all participants with PVs. In recent studies, the percentage of patients with previously undescribed PVs was 7–19% [9, 43, 44] that means that the most frequent PVs are already known in Russia. However, many new mutations can be discovered in subsequent studies, and we could reveal new highly recurrent PVs, including BRCA2 c.5286 T > G, previously identified in ovarian cancer patients and thought rare [45]. The haplotype analysis of these PV carriers showed that c.5286 T > G appeared to have arisen twice as late as the BRCA1 c.5266dup variant, but more PV carriers are necessary to unravel the time and place of its origin.
Such a high number of previously undescribed PVs observed in new studies suggests that positive selection in BRCA1 and BRCA2 genes may still be operating on these genes [46] and new pathogenic variants might be occurring in different populations nowadays leading to an increased risk of breast and ovarian cancer [47]. In addition to clarifying the highly recurrent PV frequencies, we have identified several previously undescribed PVs which occurrence exceeded the BRCA2 c.5946delT variant frequency (c.5152 + 1G > T, c.1687C > T, c.4689C > G, c.1510del, and c.2285_2286del in the BRCA1 gene; and c.5286 T > G, c.2808_2811del, c.3847_3848del, c.658_659del, and c.7879A > T in the BRCA2 gene). The total frequency of these PVs is about 73%. In addition, we identified a previously unknown PV BRCA1 c.2285_2286del that was detected in eight participants. At the same time, such a high frequency of unique PVs indicates the importance of sequencing the whole-coding sequences of the BRCA1/2 genes.
The study has some limitations. First, we could not collect detailed clinical data (e.g., age at onset of the disease or response to targeted therapy) due to difficulties with access to this type of information. Such data could allow testing the hypothesis that tumors with distinct PVs in BRCA1 and BRCA2 genes may have a different sensitivity to chemotherapy or therapy with PARP inhibitors [29, 48]. Mechanisms of such difference in the sensitivity or resistance are likely to be related to the ability of genes to form different isoforms including or skipping exon with a PV [49, 50]. At the same time, using data on ages of 265 patients at the moment of testing BRCA1/2 genes, we indirectly confirmed that BRCA1 PV carriers have earlier disease onset than patients with a BRCA2 PV [29]. Secondly, we did not identify ethnic groups for the samples, since this information is not mandatory for medical registration and registed rarely by clinicians. Moreover, this data are commonly collected with questionnaires, and although there are more than 100 different ethnic groups in Russia most of Russia inhabitants consider themselves Russians, regardless of their real ethnicity. One more reason is that many people in Russia are descendants of several ethnic groups, and it could not be determined without specific genetic analysis. Therefore, to identify PVs specific for a particular ethnic group in Russia, a separate study should be carried out. For these reasons, we studied only the geographical distribution of PVs identified that could be useful for organizing screening programs of healthy populations in whole country or in certain regions as it was suggested previously [51].
A more important limitation of this study was the absence of CNV data. In some countries, large rearrangements (mainly equal to copy number variations, CNVs) are known to be recurrent in BRCA1/2 genes, e.g., in Mexico (BRCA1 ex9-12del) [52], and this limitation should be eliminated in the future.
In conclusion, this study showed the real pathogenic and likely pathogenic germline variant occurrence in BRCA1 and BRCA2 genes in ovarian cancer patients of Russia; revealed new founder PVs, suggested the time of BRCA2 c.5286 T > G origin; and discovered 44 previously undescribed PVs. All these new data are useful for identifying patients at high risk of breast or ovarian cancer and for studying the spread of various PVs not only in Russia, but also in other countries.
Data availability
Data supporting the findings of this study are available within the article and its supplementary materials.
References
Amin N, Chaabouni N, George A (2020) Genetic testing for epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol 65:125–138. https://doi.org/10.1016/J.BPOBGYN.2020.01.005
George A, Kaye S, Banerjee S (2017) Delivering widespread BRCA testing and PARP inhibition to patients with ovarian cancer. Nat Rev Clin Oncol 14:284–296
Boussios S, Abson C, Moschetta M et al (2020) Poly (ADP-Ribose) polymerase inhibitors: Talazoparib in ovarian cancer and beyond. Drugs R D 20:55–73. https://doi.org/10.1007/s40268-020-00301-8
Santonocito C, Rizza R, Paris I et al (2020) Spectrum of germline BRCA1 and BRCA2 variants identified in 2351 ovarian and breast cancer patients referring to a reference cancer hospital of rome. Cancers (Basel). https://doi.org/10.3390/CANCERS12051286
You Y, Li L, Lu J et al (2020) Germline and somatic BRCA1/2 mutations in 172 Chinese women with epithelial ovarian cancer. Front Oncol. https://doi.org/10.3389/FONC.2020.00295
Kim H, Cho D-Y, Choi DH et al (2012) Characteristics and spectrum of BRCA1 and BRCA2 mutations in 3,922 Korean patients with breast and ovarian cance. Breast Cancer Res Treat 134:1315–1326. https://doi.org/10.1007/s10549-012-2159-5
Janavičius R, Rudaitis V, Mickys U et al (2014) Comprehensive BRCA1 and BRCA2 mutational profile in Lithuania. Cancer Genet 207:195–205. https://doi.org/10.1016/j.cancergen.2014.05.002
Kim YC, Zhao L, Zhang H et al (2016) Prevalence and spectrum of BRCA germline variants in mainland Chinese familial breast and ovarian cancer patients. Oncotarget 7:9600–9612. https://doi.org/10.18632/oncotarget.7144
Heramb C, Wangensteen T, Grindedal EM et al (2018) BRCA1 and BRCA2 mutation spectrum an update on mutation distribution in a large cancer–genetics clinic in Norway. Hered Cancer Clin Pract 16:3. https://doi.org/10.1186/s13053-017-0085-6
Wiesman C, Rose E, Grant A et al (2017) Experiences from a pilot program bringing BRCA1/2 genetic screening to the US Ashkenazi Jewish population. Genet Med 19:529–536. https://doi.org/10.1038/gim.2016.154
Sokolenko AP, Sokolova TN, Ni VI et al (2020) Frequency and spectrum of founder and non-founder BRCA1 and BRCA2 mutations in a large series of Russian breast cancer and ovarian cancer patients. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-020-05827-8
Kechin A, Khrapov E, Boyarskikh U et al (2018) BRCA-analyzer: Automatic workflow for processing NGS reads of BRCA1 and BRCA2 genes. Comput Biol Chem 77:297–306. https://doi.org/10.1016/j.compbiolchem.2018.10.012
Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38:e164. https://doi.org/10.1093/nar/gkq603
Robinson JT, Thorvaldsdóttir H, Winckler W et al (2011) Integrative genomics viewer. Nat Biotechnol 29:24–26. https://doi.org/10.1038/nbt.1754
Freeman PJ, Hart RK, Gretton LJ et al (2018) Variantvalidator: accurate validation, mapping, and formatting of sequence variation descriptions. Hum Mutat 39:61. https://doi.org/10.1002/HUMU.23348
Virtanen P, Gommers R, Oliphant TE et al (2020) SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods 17(3):261–272. https://doi.org/10.1038/s41592-019-0686-2
Kechin A, Borobova V, Boyarskikh U et al (2020) NGS-PrimerPlex: high-throughput primer design for multiplex polymerase chain reactions. PLoS Comput Biol 16:e1008468. https://doi.org/10.1371/journal.pcbi.1008468
1000 Genomes Project Consortium {fname}, Abecasis GR, Auton A et al (2012) An integrated map of genetic variation from 1,092 human genomes. Nature 491:56–65. https://doi.org/10.1038/nature11632
Browning SR, Browning BL (2007) Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am J Hum Genet 81:1084–1097. https://doi.org/10.1086/521987
Gandolfo LC, Bahlo M, Speed TP (2014) Dating rare mutations from small samples with dense marker data. Genetics 197:1315–1327. https://doi.org/10.1534/genetics.114.164616
Rebbeck TR, Mitra N, Wan F et al (2015) Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA 313:1347–1361. https://doi.org/10.1001/jama.2014.5985
Janavičius R (2010) Founder BRCA1/2 mutations in the Europe: implications for hereditary breast-ovarian cancer prevention and control. EPMA J 1:397. https://doi.org/10.1007/S13167-010-0037-Y
Karami F, Mehdipour P (2013) A comprehensive focus on global spectrum of BRCA1 and BRCA2 mutations in breast cancer. Biomed Res Int 2013:1–21. https://doi.org/10.1155/2013/928562
Iyevleva AG, Suspitsin EN, Kroeze K et al (2010) Non-founder BRCA1 mutations in Russian breast cancer patients. Cancer Lett 298:258–263. https://doi.org/10.1016/j.canlet.2010.07.013
Infante M, Duran M, Acedo A et al (2013) The highly prevalent BRCA2 mutation c.2808_2811del (3036delACAA) is located in a mutational hotspot and has multiple origins. Carcinogenesis 34:2505–2511. https://doi.org/10.1093/carcin/bgt272
Incorvaia L, Fanale D, Badalamenti G et al (2020) Hereditary breast and ovarian cancer in families from southern Italy (sicily)—prevalence and geographic distribution of pathogenic variants in BRCA1/2 genes. Cancers 12:1158. https://doi.org/10.3390/CANCERS12051158
Kluz T, Jasiewicz A, Marczyk E et al (2018) Frequency of BRCA1 and BRCA2 causative founder variants in ovarian cancer patients in South-East Poland. Hered Cancer Clin Pract 16:6. https://doi.org/10.1186/s13053-018-0089-x
Jakimovska M, Kostovska IM, Popovska-Jankovic K et al (2018) BRCA1 and BRCA2 germline variants in breast cancer patients from the Republic of Macedonia. Breast Cancer Res Treat 168(3):745–753. https://doi.org/10.1007/S10549-017-4642-5
Hollis RL, Churchman M, Gourley C (2017) Distinct implications of different BRCA mutations: efficacy of cytotoxic chemotherapy, PARP inhibition and clinical outcome in ovarian cancer. Onco Targets Ther 10:2539–2551. https://doi.org/10.2147/OTT.S102569
Hamel N, Feng BJ, Foretova L et al (2011) On the origin and diffusion of BRCA1 c.5266dupC (5382insC) in European populations. Eur J Hum Genet 19:300–306. https://doi.org/10.1038/ejhg.2010.203
Sokolenko AP, Bogdanova N, Kluzniak W et al (2014) Double heterozygotes among breast cancer patients analyzed for BRCA1, CHEK2, ATM, NBN/NBS1, and BLM germ-line mutations. Breast Cancer Res Treat 145:553–562. https://doi.org/10.1007/s10549-014-2971-1
Snigireva G, Rumyantseva V, Novikova E et al (2019) Algorithm of molecular genetic investigation to identify hereditary BRCA-associated breast cancer. Alʹm klin med. https://doi.org/10.18786/2072-0505-2019-47-002
Rebbeck TR, Friebel TM, Friedman E et al (2018) Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat 39:593–620. https://doi.org/10.1002/humu.23406
Barnes-Kedar I, Bernstein-Molho R, Ginzach N et al (2018) The yield of full BRCA1/2 genotyping in Israeli high-risk breast/ovarian cancer patients who do not carry the predominant mutations. Breast Cancer Res Treat 172:151–157. https://doi.org/10.1007/s10549-018-4887-7
Machackova E, Foretova L, Lukesova M et al (2008) Spectrum and characterisation of BRCA1 and BRCA2 deleterious mutations in high-risk Czech patients with breast and/or ovarian cancer. BMC Cancer 8:140. https://doi.org/10.1186/1471-2407-8-140
Solano AR, Aceto GM, Delettieres D et al (2012) BRCA1 And BRCA2 analysis of Argentinean breast/ovarian cancer patients selected for age and family history highlights a role for novel mutations of putative south-American origin. Springerplus 1:20. https://doi.org/10.1186/2193-1801-1-20
Suspitsin EN, Sherina NY, Ponomariova DN et al (2009) High frequency of BRCA1, but not CHEK2 or NBS1 (NBN), founder mutations in Russian ovarian cancer patients. Hered Cancer Clin Pract 7:1–7. https://doi.org/10.1186/1897-4287-7-5
Gomes R, Soares BL, Felicio PS et al (2020) Haplotypic characterization of BRCA1 c.5266dupC, the prevailing mutation in Brazilian hereditary breast/ovarian cancer. Genet Mol Biol. https://doi.org/10.1590//1678-4685-gmb-2019-0072
Janavičius R, Rudaitis V, Feng BJ et al (2013) Haplotype analysis and ancient origin of the BRCA1 c.4035delA Baltic founder mutation. Eur J Med Genet 56:125–130. https://doi.org/10.1016/j.ejmg.2012.12.007
Nguyen-Dumont T, Karpinski P, Sasiadek MM et al (2020) Genetic testing in Poland and Ukraine: should comprehensive germline testing of BRCA1 and BRCA2 be recommended for women with breast and ovarian cancer? Genet Res (Camb). https://doi.org/10.1017/S0016672320000075
Kowalik A, Siołek M, Kopczyński J et al (2018) BRCA1 founder mutations and beyond in the polish population: a single-institution BRCA1/2 next-generation sequencing study. PLoS ONE. https://doi.org/10.1371/journal.pone.0201086
Vézina H, Durocher F, Dumont M et al (2005) Molecular and genealogical characterization of the R1443X BRCA1 mutation in high-risk French-Canadian breast/ovarian cancer families. Hum Genet 117(2):119–132. https://doi.org/10.1007/S00439-005-1297-9
Rashid MU, Muhammad N, Naeemi H et al (2019) Spectrum and prevalence of BRCA1/2 germline mutations in Pakistani breast cancer patients: results from a large comprehensive study. Hered Cancer Clin Pract 17:1–13. https://doi.org/10.1186/s13053-019-0125-5
Bu H, Chen J, Li Q et al (2019) BRCA mutation frequency and clinical features of ovarian cancer patients: a report from a chinese study group. J Obstet Gynaecol Res 45:2267–2274. https://doi.org/10.1111/jog.14090
Berlev IV, Urmancheeva AF, Imyanitov EN et al (2018) The clinical course of ovarian cancer in a patient with the rare c.5286T>G (p.Y1762X) mutation in the BRCA2 gene. DoctorRu 154:43–46. https://doi.org/10.31550/1727-2378-2018-154-10-43-46
Lou DI, McBee RM, Le UQ et al (2014) Rapid evolution of BRCA1 and BRCA2 in humans and other primates. BMC Evol Biol 14:1–13. https://doi.org/10.1186/1471-2148-14-155
Miki Y, Swensen J, Shattuck-Eidens D et al (1994) A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266:66–71
Yang D, Khan S, Sun Y et al (2011) Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA 306:1557–1565. https://doi.org/10.1001/JAMA.2011.1456
Dimitrova D, Ruscito I, Olek S et al (2016) Germline mutations of BRCA1 gene exon 11 are not associated with platinum response neither with survival advantage in patients with primary ovarian cancer: understanding the clinical importance of one of the biggest human exons. A study of the tumor bank ovarian cancer (TOC) consortium. Tumour Biol 37:12329–12337. https://doi.org/10.1007/S13277-016-5109-8
Drost R, Dhillon KK, van der Gulden H et al (2016) BRCA1185delAG tumors may acquire therapy resistance through expression of RING-less BRCA1. J Clin Invest 126:2903–2918. https://doi.org/10.1172/JCI70196
Ishkineeva FF, Ozerova KA, Kaveeva AD, Husnullina ES (2018) The population need in genetic tests for predisposition to breast cancer. Probl Sotsialnoi Gig Zdravookhranenniiai Istor Med. 26:276–281. https://doi.org/10.32687/0869-866X-2018-26-5-276-281
Gallardo-Rincón D, Álvarez-Gómez RM, Montes-Servín E et al (2020) Clinical evaluation of BRCA1/2 mutation in mexican ovarian cancer patients. Transl Oncol 13:212–220. https://doi.org/10.1016/j.tranon.2019.11.003
Acknowledgements
The authors greatly appreciate the assistance of the inter-regional non-governmental organization “Society of molecular geneticists in oncology and oncohematology.”
Funding
The study was supported partially under Russian State-funded budget project 0245-2021-0006 “Fundamentals of Health Preservation” and within the state assignment of the Ministry of Science and Higher Education of the Russian Federation for RCMG.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. AK, UB, AB, AT, SK, AZ, EK, SS, OM: material preparation, data collection, and analysis were performed. The first draft of the manuscript was written by AK, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The study was approved by the local medical ethics committee of the Institute of Chemical Biology and Fundamental Medicine of the Siberian Branch of the Russian Academy of Sciences, Ethics approval No 11.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kechin, A., Boyarskikh, U., Barinov, A. et al. A spectrum of BRCA1 and BRCA2 germline deleterious variants in ovarian cancer in Russia. Breast Cancer Res Treat 197, 387–395 (2023). https://doi.org/10.1007/s10549-022-06782-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06782-2