Abstract
Purpose
Vaccination is an essential strategy to prevent infection in the SARS-CoV-2 pandemic. However, there are concerns about vaccine efficacy and the impact of vaccination on cancer treatment. Additionally, the emergence of novel variants may affect vaccination efficacy. This multi-center, prospective, observational study investigated the efficacy and impact of vaccination against SARS-CoV-2 variants on treatment among breast cancer patients in Japan.
Methods
Patients with breast cancer scheduled to be vaccinated with the SARS-CoV-2 vaccine from May to November 2021 were prospectively enrolled (UMIN000045527). They were stratified into five groups according to their cancer treatment: no treatment, hormone therapy, anti-human epidermal growth factor receptor (HER)2 therapy, chemotherapy, and cyclin-dependent kinase 4/6 (CDK4/6) inhibitor. Serum samples for assessing serological responses were collected before the first vaccination and after the second vaccination.
Results
Eighty-five breast cancer patients were included. The overall seroconversion rate after second vaccination was 95.3% and the lowest seroconversion rate was 81.8% in the patients under chemotherapy. The overall positivity rate of neutralizing antibodies against the wild-type, α, Δ, κ, and omicron variants were 90.2%, 81.7%, 96.3%, 84.1%, and 8.5%, respectively. Among the patients under chemotherapy or CDK4/6 inhibitors, various degrees of decreased neutralizing antibody titers against SARS-CoV-2 variants were observed. Withdrawal or reduction of systemic therapy because of vaccination was observed in only one patient.
Conclusion
Our data support SARS-CoV-2 vaccination for breast cancer patients. However, a reduction in neutralizing antibody titers was suggested during chemotherapy and CDK4/6 inhibitors, raising concerns about the impact on long-term infection prevention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pandemic of acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the resulting worldwide spread of coronavirus disease 2019 (COVID-19) promoted the development of novel variants for host immune response escape. The development of vaccines against SARS-CoV-2 has had a profound impact on the prevention of infection. The pivotal phase 3 trials of BNT162b2 vaccine and mRNA-1273 vaccine against SARS-CoV-2 demonstrated 94.1%–95.0% efficacy at preventing COVID-19 including severe disease [1, 2]. However, there is emerging concern that SARS-CoV-2 variants with mutations in the S-protein (S) receptor-binding domain (RBD) of SARS-CoV-2, the target site of the vaccine, are more resistant to neutralizing antibodies [3, 4].
Although patients with cancer represent an important vulnerable group because of their immunological deficiency, the pivotal trials of SARS-CoV-2 vaccines contained few patients with cancer, and data on the efficacy and immune response to SARS-CoV-2 vaccination in these populations are lacking. While available studies demonstrated that the immune response to COVID-19 vaccines was attenuated in patients with hematological malignancies compared with healthy individuals exhibiting lower rates of seroconversion [5,6,7,8,9], the effect of cancer treatment, especially cytotoxic chemotherapy, among patients with solid malignant tumors is controversial. Currently, breast cancer patients are treated with various types of therapy including endocrine therapy, chemotherapy, anti-human epidermal growth factor receptor 2 (HER2) therapy, cyclin-dependent kinase 4/6 (CDK4/6) inhibitors, immune check point inhibitors, and other molecular-targeted therapy. The effect of these various systemic therapies on seroconversion and neutralizing antibody concentration is uncertain. The emergence of novel variants also obscures the efficacy of vaccination. Although there has been a report of decreased antibody titers against the omicron variant in patients with cancer [10], the effect of treatment on neutralizing antibody titers against each variant of SARS-CoV-2 remains unclear.
The adequate timing of SARS-CoV-2 vaccination has been identified in terms of both the immunological response and the effect of treatment schedule. While local and systemic adverse effects of SARS-CoV-2 vaccination are common but are mainly low grade and of short duration [8, 11], these adverse effects can have a negative impact on cancer therapy by interrupting or delaying the treatments due to adverse events themselves or concerns regarding them. Especially in early breast cancer, low relative dose intensity of chemotherapy reductions has a negative impact on survival [12,13,14]. However, the effect of SARS-CoV-2 vaccination on clinical decisions for cancer treatment has been poorly studied. Considering the appropriate timing of vaccination, it is necessary to study the overall immunological response to the vaccine and its impact on treatment.
Here, we conducted a multi-center prospective observational study to reveal the efficacy and impact of vaccination against SARS-CoV-2 on cancer treatment among breast cancer patients. We also demonstrated the differences in neutralizing activity in each cancer treatment group against B.1.1.7 (α), B.1.617.2 (Δ), B.1.617.1 (κ), and B.1.1.529 (omicron) variants compared with wild-type (WT) SARS-CoV-2 variant.
Methods
Study design
This prospective observational study was designed to determine the serological change in IgG against S-protein antigen after SARS-CoV-2 vaccination and the effect of vaccination on cancer treatment plans among breast cancer patients (UMIN000045527). Eligible criteria were as follows: (1) patients with histologically confirmed breast cancer; (2) age above 20 years; (3) eligibility for SARS-CoV-2 vaccination; and (4) patients without any SARS-CoV-2 vaccines before participation in this study. Patients known to be previously infected with COVID-19 virus were excluded.
Participants were recruited at Nagoya City University Hospital (Aichi, Japan), Nagoya City University West Medical Center (Aichi, Japan), Nagoya City University East Medical Center (Aichi, Japan), Sapporo Medical University Hospital (Hokkaido, Japan), Akita University Hospital (Akita, Japan), Mie University Hospital (Mie, Japan), and Okayama University Hospital (Okayama, Japan) between June 2021 and November 2021. Participants were assigned to one of four groups according to the treatment that they were receiving at the time of consent: no treatment, endocrine therapy (without any molecular-targeted therapies), CDK4/6 inhibitors, or other treatments (including chemotherapy, anti-HER2 therapy, and other molecular-targeted therapies). The other treatment group was finally separated into two groups that received only anti-HER2 therapy (anti-HER2 therapy group) and those that did not (chemotherapy group) at analysis. The total estimated enrollments were 100 patients. Clinical data and serum samples were collected at baseline (within 1 month before the date of first vaccination) and at post-vaccination (28 ± 14 days after the second vaccination). The primary outcome for this analysis was the concentration of anti-SARS-CoV-2 S-RBD IgG in patients at 28 days following the second dose of vaccine. The secondary outcomes were seroconversion rates and concentrations of anti-SARS-CoV-2 IgG in each group, SARS-CoV-2 neutralizing antibody titers against each SARS-CoV-2 variants (WT, α, Δ, κ, and omicron), and the rate of treatment delay or reduction from first vaccination to post-vaccination.
Patient data
Demographic, epidemiological, and clinical data including breast cancer stage and treatment history, and type of vaccine were collected from the electronic patient records. Concomitant medications were collected for corticosteroids. When a physician decided to delay and reduce the treatment dose, the reasons for this delay and reduction were recorded from the following options: (1) concerns about possible adverse events of vaccination; (2) adverse events of vaccination; (3) adverse events of cancer treatment; and (4) others. At the end of follow-up, the delay or reduction of treatments was also recorded. To exclude the selective outcome reporting bias, the data of treatment delay and reduction were obtained prospectively.
Serum isolation
Ten milliliters of blood were collected in serum coagulation tubes for serum isolation and stored at 4 °C until processing. All samples were processed within 24 h. Before processing, tubes were brought to room temperature. Tubes were centrifuged for 10 min at 3000×g at room temperature. Serum was separated from the clotted portion, aliquoted, and stored at − 20 °C.
Anti-SARS-CoV-2 S-RBD IgG enzyme-linked immunosorbent assay (ELISA)
To measure the IgG tilter at baseline and post-vaccination, IgG for SARS-CoV-2 S-RBD in serum was measured with an Anti-SARS-CoV-2 S-RBD protein Human IgG ELISA kit (Proteintech, USA) according to the manufacturer’s protocol. Briefly, each standard and diluted sample (1:100) with sample diluent buffer was added to the wells in duplicate and plates were incubated at room temperature for 30 min. After washing five times with washing buffer, plates were incubated with horseradish peroxidase (HRP)-conjugated anti-human IgG secondary antibody (100 μl) at room temperature for 30 min. After washing five times with washing buffer, substrate tetramethylbenzidine (100 μl) was added to each well, and plates were incubated at room temperature in the dark for 10 min in the dark. Stop solution (100 μl) was added to stop the reaction. Finally, absorbance was measured at 450 nm in an ELISA reader (Infinite F50, Tecan). When the calculated value was below the detection sensitivity, the concentration was considered as 0 ng/ml. The definition of seroconversion was positive antibody concentration above the detection sensitivity after vaccination from negative at baseline.
SARS-CoV-2 neutralizing antibody titer against SARS-CoV-2 variants
Neutralizing antibody titers elicited by vaccination were analyzed with a SARS-CoV-2 Neutralizing Antibodies Detection Kit (AdipoGen): wild type, B.1.1.7 variant (α), B.1.617.2 variant (Δ), and B.1.617.1 variant (κ); and Anti-SARS-CoV-2 (B.1.529) Neutralizing Antibody Titer Serologic Assay Kit (ACROBiosystems, USA): B.1.1.529 variant (omicron). Neutralizing antibody titers against each variant were evaluated using patient serum according to the manufacturer’s protocol. Briefly, sera were diluted in preparation reagents (1:10). Supplied positive and negative control samples and serum (100 μl) were then added to wells coated with each variant RBD protein in duplicate and incubated for 1 h at 37 °C. After washing five times with washing buffer, plates were incubated with angiotensin-converting enzyme (ACE) 2 (human)-HRP for 1 h at 37 °C. After washing again five times with washing buffer, substrate tetramethylbenzidine (100 μl) was added to each well and plates were incubated at room temperature for 10 min in the dark. Stop solution (100 μl) was added to halt the reaction. Finally, absorbance was measured at 450 nm in an ELISA reader (Infinite F50, Tecan). The inhibition rate as neutralizing antibody titer was calculated. The cutoff was defined as above 20%, as indicated in the manufacturer’s protocol.
Statistical analysis
χ2 test for categorical variables or the Kruskal–Wallis test for continuous variables were performed to determine statistical differences for patient characteristics. Kruskal–Wallis test was performed to compare the concentrations of antibodies and the titers of neutralizing antibodies against SARS-CoV-2 variants in each group. Spearman’s correlation test was applied for the correlation between white blood cell counts or lymphocyte counts and the concentration of SARS-CoV-2 antibody. The patients for which paired serum samples including loss of follow-up were not available were excluded from all the analyses. The patients who are seropositive of the baseline serum sample were also excluded from all the analyses. P values < 0.05 were considered significant. All tests were performed in a two-sided manner. Data analysis and statistical analysis were performed in GraphPad Prism 9.
Results
Patients’ characteristics
During the study period, a total of 98 breast cancer patients with SARS-CoV-2 vaccination were enrolled. According to the protocol, 13 patients were excluded (death during the study period, n = 1; lacking samples at the time point of second vaccination, n = 2; deviation in timing of sample collection, n = 9; and serological positivity of anti-SARS-CoV-2 IgG at the baseline, n = 1), and, finally, 85 patients were analyzed. Table 1 summarizes the patients’ characteristics. The median age was 62.5 years (range, 21–82 years). The median days between second vaccination and the time point of post-vaccination was 30 days (range, 15–44 days), and the median days between the first and second vaccinations and the date of last treatment were both 0 days (ranges, 0–18 and 0–32 days, respectively). Among all patients, 55.3% (n = 47) were early stage and 44.7% (n = 38) were advanced stage or had metastatic disease, and the CDK4/6 inhibitor, chemotherapy, and anti-HER2 therapy groups had advanced or metastatic disease more frequently than the no-treatment and endocrine therapy groups. The types of SARS-CoV-2 vaccine administered were BNT162b2 (n = 65, 76.5%) and mRNA-1273 vaccine (n = 3, 3.5%), and 21.2% (n = 18) were undetermined. The patients were assigned to the no-treatment (n = 5), endocrine therapy (n = 30), CDK4/6 inhibitor (n = 14), and other treatment (n = 36) groups including the chemotherapy group (n = 21) and the anti-HER2 therapy group (n = 15). Among the chemotherapy group, anthracycline-based therapy (n = 1, 4.8%), taxane (n = 11, 52.4%), cyclophosphamide–methotrexate–fluorouracil (CMF) (n = 3, 14.3%), oral 5-fluorouracil (n = 6, 28.6%), and combination with anti-HER2 therapy (n = 7, 33.3%) were administered. During the study period, 16.5% of patients (n = 14) received corticosteroids, with significantly more patients in the chemotherapy group receiving them than the other groups. The white blood cell and lymphocyte counts at the baseline were significantly different: the white blood cell counts were lower in the CDK4/6 inhibitor group (median, 3100 counts/μl) and the lymphocyte counts were lower in the chemotherapy group (median, 692 counts/μl).
Antibody responses to vaccination
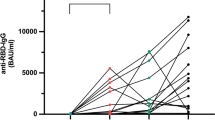
One patient was serologically positive for anti-SARS-CoV-2 S-RBD immunoglobulin G (anti-SARS-CoV-2 IgG) at baseline, suggesting that this patient was previously infected without a history of infection in our cohort. Among 85 eligible patients, the median antibody concentration among all patients was 19,984 ng/μl (range, 0.0–99,405 ng/μl) at the time point of post-vaccination. The median antibody concentration at the time point of post-vaccination in the no-treatment, endocrine therapy, CDK4/6 inhibitor, chemotherapy, and anti-HER2 therapy groups was 31,814 ng/μl (range, 20,484–47,496 ng/μl), 29,744 ng/μl (range, 1,455–99,405 ng/μl), 14,608 ng/μl (range, 2,574–61,098 ng/μl), 3,894 ng/μl (range, 0–91,912 ng/μl), and 24,689 ng/μl (range, 3,292–94,790 ng/μl), respectively (Fig. 1a). The antibody concentration in the chemotherapy group was significantly decreased compared with that in the no-treatment group (p = 0.02). The total seroconversion rate was 95.3%. The seroconversion rate in the no-treatment, endocrine therapy, CDK4/6 inhibitor, chemotherapy, and anti-HER2 therapy groups was 100%, 100%, 100%, 81.8%, and 100%, respectively (Fig. 1b). Breast cancer disease status at vaccination had no impact on the IgG concentration after vaccination (p = 0.63; Fig. 1c). To investigate the effect of corticosteroid administration in the chemotherapy group during the study period, we compared anti-SARS-CoV-2 IgG concentration with and without corticosteroids. There was no difference in the concentration between patients with and without corticosteroids in the chemotherapy group (p = 0.21; Fig. 1d). Spearman’s correlation test showed a positive correlation between total white blood cell count (r = 0.33, p = 0.0028; Fig. 2a) and lymphocyte counts (r = 0.23, p = 0.042; Fig. 2b) and the concentration of anti-SARS-CoV-2 IgG.
Anti-SARS-CoV-2 IgG concentration after second vaccination of the treatment groups. Anti-SARS-CoV-2 IgG concentrations before vaccination and post-vaccination were measured by ELISA in the no-treatment (n = 5), endocrine therapy (n = 30), CDK4/6 inhibitor (n = 14), chemotherapy (n = 21), and anti-HER2 therapy (n = 15) treatment groups (a). Anti-SARS-CoV-2 IgG seroconversion rates of the treatment groups (b). Anti-SARS-CoV-2 IgG concentration by disease stage treatment lines for advanced/metastatic disease (c). Anti-SARS-CoV-2 IgG by administration of corticosteroids in the chemotherapy group (d). Significance was tested by two-sided Kruskal–Wallis test; p < 0.05 was considered significant (ns; not significant, *p < 0.05)
Correlation between peripheral white blood cell counts and lymphocyte counts and concentration of SARS-CoV-2 antibody in the CDK4/6 inhibitor and chemotherapy groups. Correlation between white blood cell counts (a) and lymphocyte counts (b) (counts/μl) before vaccination and concentration of SARS-CoV-2 IgG (ng/μl) at the time point of post-vaccination. Spearman’s correlation test was applied for the correlation between white blood cell or lymphocyte counts and concentration of SARS-CoV-2 antibody; p < 0.05 was considered significant (ns; not significant, *p < 0.05, **p < 0.01)
Neutralizing antibody against SARS-CoV-2 variants
Functional humoral response after vaccination was assessed by evaluating neutralizing antibody against SARS-CoV-2 variants from each group (no treatment, n = 5; endocrine therapy, n = 26; CDK4/6 inhibitor, n = 15; chemotherapy, n = 21; and anti-HER2 therapy, n = 15). Overall, we observed that neutralizing antibody titers against α, Δ, κ, and omicron were significantly lower than WT (WT vs α, p < 0.0001; WT vs Δ, p = 0.012; WT vs κ, p = 0.009; and WT vs omicron, p < 0.0001) (Fig. 3a). The neutralizing antibody titers against each variant in the endocrine therapy group and the anti-HER2 therapy group were comparable to the no-treatment group (Fig. 3b–f). There were significant decreases in the antibody titers against WT (p = 0.008) and α (p = 0.006) in the CDK4/6 inhibitor group compared with those in the no-treatment group (Fig. 3b and Fig. 3c), while there was a trend toward lower titers against other variants. In the chemotherapy group, the neutralizing antibody titers against WT (p = 0.001; Fig. 3b), α (p < 0.001; Fig. 3c), and κ (p = 0.03; Fig. 3e) were significantly lower than the no-treatment group. Overall positivity rates of neutralizing antibodies against WT, α, Δ, κ, and omicron were 90.2%, 81.7%, 96.3%, 84.1%, and 8.5%, respectively. Among 21 patients in the chemotherapy group, neutralizing antibody titers against WT, α, Δ, κ, and omicron were above the cutoff in 66.7%, 66.7%, 81.0%, 47.6%, and 0.0% patients, respectively. All patients in the no-treatment, endocrine therapy, CDK4/6 inhibitor, and anti-HER2 therapy groups showed positive neutralizing antibodies against WT, α, Δ and κ. The positive rates of neutralizing antibodies against omicron in the no-treatment, endocrine therapy, CDK4/6 inhibitor, and anti-HER2 therapy groups were 0.0%, 3.8%, 6.25%, and 33.3%, respectively (Fig. 3f).
Neutralizing antibody titers against SARS-CoV-2 variants after second vaccination of the treatment groups. Comparison of neutralizing antibody titers against each variant compared with WT (n = 82) (a). Comparison of neutralizing antibody titers against WT (b), α (c), Δ (d), κ (e), and omicron (f) in each group (no treatment, n = 5; endocrine therapy, n = 26; CDK4/6 inhibitor, n = 15; chemotherapy, n = 21; and anti-HER2 therapy, n = 15) at the time point of post-vaccination. Neutralizing antibody titers are represented as the rate (%) of reaction inhibition between RBD protein of each variant and ACE-HRP with patient serum by ELISA. The dotted line at 20% denotes the cutoff point of neutralizing antibody. Neutralizing antibody-positivity rates shown under each symbol for the group. Significance in (a) to (f) was tested compared with the no-treatment group by two-sided Kruskal–Wallis test; p < 0.05 was considered significant (ns; not significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). WT; wild type, α; α variant, δ; Δ variant, κ; κ variant, and ο; omicron variant.
Treatment delay and reduction during peri-SARS-CoV-2 vaccination
Treatment delay and reduction information were obtained from patients who received systemic therapy (Table 2). Treatment delay or reduction between the study inclusion date to post-vaccination was observed in 10.6% of patients (n = 9) because of concerns about possible vaccine adverse events (n = 1, 1.2%), adverse events from the treatment itself (n = 7, 8.2%), and treatment delay due to national holidays (n = 1, 1.2%). There was no patient who experienced a treatment delay or reduction because of adverse events resulting from vaccination. The rates of treatment delay or reduction in the endocrine therapy, CDK4/6 inhibitor, chemotherapy, and anti-HER2 therapy groups were 0.0%, 42.9%, 14.3%, and 0.0%, respectively.
Discussion
Our study demonstrated a high seroconversion rate of 95.3% after second SARS-CoV-2 vaccination among breast cancer patients. However, the lower titer against SARS-CoV-2 variants was also observed in the patients under chemotherapy and CDK4/6 inhibitors compared with patients with no systemic treatment. To our knowledge, this is the first report that demonstrated decreased neutralizing antibody titers in patients who were being treated with CDK4/6 inhibitors, even though the seroconversion rate was comparable to patients without systemic therapy. Additionally, the different SARS-CoV-2 variants affected the neutralizing antibody titers after vaccination, and, notably, two doses of the current vaccine failed to elicit effective neutralizing antibody against omicron regardless of their treatment.
Although our results of the seroconversion rates after SARS-CoV-2 vaccination were comparable to previous reports [6, 15, 16], the titer of neutralizing antibodies against several variants in the chemotherapy and CDK4/6 inhibitors groups was lower than in other groups, even in seropositive patients. In an in vitro study, serum antibodies from vaccinated individuals showed good recognition of most SARS-CoV-2 variants, but reduced inhibition of RBD-ACE2 binding [17]. Additionally, consistent with our results, the CAPTURE study demonstrated that seroconversion showed poor concordance with neutralizing antibody against SARS-CoV-2 variants among patients with cancer [18]. While good concordance was observed between the S-protein-reactive antibodies and neutralizing antibody against WT, there was discordance in the case of SARS-CoV-2 variants; for example, 55% patients with detectable anti-S-protein antibodies had no detectable neutralizing antibody against Δ after vaccination [18]. The impact of chemotherapy on vaccination effects is still controversial. Chemotherapy attenuated immune responses after SARS-CoV-2 vaccination in some reports [7, 19]. However, some studies reported that chemotherapy did not affect the immune response to vaccination [16, 18]. Moreover, the relationship between immune response to vaccine and CDK4/6 inhibitors has been poorly investigated. One study showed that immune response did not differ between patients receiving CDK4/6 inhibitors and healthy individuals, but the evaluation was undertaken after the first vaccination [20]. Both chemotherapy and CDK4/6 inhibitors have common adverse events of leukopenia and lymphopenia, which can lead to attenuated immune responses to vaccination. Our study also demonstrated a positive correlation between anti-SARS-CoV-2 antibody concentration and the numbers of white blood cells and lymphocytes. Previous reports described significant positive correlations between the antibody concentration and lymphocyte counts in patients with cancer [18] and B cell non-Hodgkin lymphoma [20], and in patients undergoing maintenance hemodialysis [21]. Of note, B cells and T cells play an important role in eliciting vaccine-specific antibody responses by vaccination. The loss of vaccine-specific antibody responses by B cell depletion has been well described in studies of patients treated with anti-CD20 therapy [22,23,24]. Among breast cancer patients, memory B cells were also transiently depleted after chemotherapy and the CD4+ T cells were severely affected in both quantity and quality for more than 5 years [25]. Additionally, a rhesus macaque model treated with doxorubicin showed that vaccine-specific memory B cells were selectively depleted, and it was implied that patients who loose protective antibodies have a diminished bone marrow plasma cell pool [26]. Considering these findings, the attenuation of neutralizing antibody titers in our study could be partially explained by the fact that chemotherapy and CDK4/6 inhibitors reduced the number of lymphocytes and the diversity of SARS-CoV-2-specific B cells, which could lead to a decrease in antigen-specific IgG and differences in neutralizing antibody titers against various variants. To uncover the underlying mechanism, further studies are needed. On the basis of our data and these previous reports, white blood cell and lymphocyte counts should be taken into account and the nadir period should be avoided when considering the timing of vaccination to elicit the best immune response to the vaccine.
Current SARS-CoV-2 vaccines were designed to target RBD in the S-protein of the B.1 variant, and the emergence of variants such as the β, γ, Δ, and omicron variants with reduced susceptibility to vaccines has raised problems [10, 18, 27,28,29]. Our results also demonstrated that decreases in neutralizing antibodies against α, Δ, κ, and omicron compared with WT. Among them, neutralizing antibody against omicron was notably quite low, even in patients with no treatment and endocrine therapy. In a study of virus neutralization assays, omicron was shown to escape antibody neutralization by the vaccination [30]. However, the contributions of a third vaccination to protection against omicron is gradually being reported [31,32,33]. Recently, the positive effect of neutralizing antibodies against omicron after third vaccination among patients with cancer was also reported even in a small population [10]. These findings supported that patients with cancer are good candidates for third booster vaccination, especially during the COVID-19 pandemic.
While the type of therapy has been shown to influence patient response to vaccination, the appropriate timing for SARS-CoV-2 vaccination in cancer patients receiving systemic therapy is uncertain. Patients who are planning to undergo chemotherapy are recommended to complete vaccination for COVID-19 at least 2 weeks before starting chemotherapy [34]. In patients participating in a phase 1 clinical trial of cytotoxic agents, vaccination 1–2 weeks before or 1–2 weeks after drug dosing, when possible, is recommended to increase the potential for the immune system to mount a response, while vaccination when available is recommended in patients with treatment of targeted therapy, hormone therapy, immunotherapy, and epigenetic therapy [35]. In addition to the problem of immune response after vaccination, delays in cancer treatment due to side effects or concerns about side effects can also be a problem, although local and systemic side effects of SARS-CoV-2 vaccination are common but are mainly low grade and of short duration and comparable in both patients with cancer and healthy individuals [8, 11]. In our study, most physicians did not issue instructions for dose reduction or withdrawal of cancer therapy before or after vaccination. Additionally, treatment delay related to adverse events of vaccination was not observed in our cohort. These findings suggested that SARS-CoV-2 vaccination can be safely performed during cancer treatment without attenuating treatment intensity.
There were some limitations in this study. First, we had no data of protection against clinical SARS-CoV-2 infection after vaccination due to the short follow-up. However, our data implied a good impact on understanding the effect of SARS-CoV-2 vaccination among patients with breast cancer because neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection [36]. Second, we focused on the humoral immune reaction using serum samples and did not evaluate B cell and T cell responses against SARS-CoV-2 after vaccination. In addition to B cells, T cells also play an important role in the protection of SARS-CoV-2 infection after vaccination. Further studies including B cell and T cell responses against SARS-CoV-2 variants after vaccination are needed. Third, there were some missing data on the manufacturer of the vaccine used. Additionally, a small number of patients were vaccinated with mRNA-1273 vaccine because mRNA-1273 was approved later than BNT162b vaccine in Japan. Thus, we did not evaluate the efficacy between BNT162b2 and mRNA-1273 vaccines. Fourth, there was no comparison with a healthy population. In this study, we compared antibody production in each treatment group, with the no-treatment group as a control. Although comparison with a healthy population would help to clarify the immunological responses among patients with breast cancer, the current results were nevertheless valuable for helping to compare the impacts of each treatment. Finally, we did not obtain details of the adverse effects of vaccination because, in many cases, vaccination was performed at local sites rather than at the hospital at which patients were being treated for cancer in Japan. Physicians’ records of the reasons for treatment can compensate for this missing data and these data suggested that low frequency of severe adverse effects is enough to cause treatment delay.
Conclusions
In conclusion, our data support SARS-CoV-2 vaccination for cancer patients being treated with systemic therapy and vaccination administered away from nadir during chemotherapy and CDK4/6 inhibitor treatment. Of note, neutralizing antibody titers against omicron were very low, even after two vaccinations among patients with or without cancer treatment. Furthermore, a reduction in neutralizing antibody titers was suggested during chemotherapy and CDK4/6 inhibitor treatment, raising concerns about the impact on long-term infection prevention. For these patients, infection-preventive behaviors should be recommended even after vaccination. They will also be good candidates for third booster vaccination.
Data availability
The data generated or analyzed during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Polack FP, Thomas SJ, Kitchin N et al (2020) Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med 383:2603–2615. https://doi.org/10.1056/NEJMoa2034577
Baden LR, El Sahly HM, Essink B et al (2021) Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384:403–416. https://doi.org/10.1056/NEJMoa2035389
McCallum M, Bassi J, De Marco A et al (2021) SARS-CoV-2 immune evasion by the B.1.427/B.1.429 variant of concern. Science 373:648–654. https://doi.org/10.1126/science.abi7994
Motozono C, Toyoda M, Zahradnik J et al (2021) SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe 29:1124-1136.e11. https://doi.org/10.1016/j.chom.2021.06.006
Thakkar A, Pradhan K, Jindal S et al (2021) Patterns of seroconversion for SARS-CoV-2 IgG in patients with malignant disease and association with anticancer therapy. Nat Cancer. https://doi.org/10.1038/s43018-021-00191-y
Addeo A, Shah PK, Bordry N et al (2021) Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell 39:1091-1098.e2. https://doi.org/10.1016/j.ccell.2021.06.009
Massarweh A, Eliakim-Raz N, Stemmer A et al (2021) Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol 7:1133–1140. https://doi.org/10.1001/jamaoncol.2021.2155
Monin L, Laing AG, Muñoz-Ruiz M et al (2021) Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol 22:765–778. https://doi.org/10.1016/S1470-2045(21)00213-8
Schmidt AL, Labaki C, Hsu C-Y et al (2021) COVID-19 vaccination and breakthrough infections in patients with cancer. Ann Oncol. https://doi.org/10.1016/j.annonc.2021.12.006
Fendler A, Shepherd STC, Au L et al (2022) Omicron neutralising antibodies after third COVID-19 vaccine dose in patients with cancer. Lancet. https://doi.org/10.1016/S0140-6736(22)00147-7
Goshen-Lago T, Waldhorn I, Holland R et al (2021) Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol 7:1507–1513. https://doi.org/10.1001/jamaoncol.2021.2675
Schraa SJ, Frerichs KA, Agterof MJ et al (2017) Relative dose intensity as a proxy measure of quality and prognosis in adjuvant chemotherapy for breast cancer in daily clinical practice. Eur J Cancer 79:152–157. https://doi.org/10.1016/j.ejca.2017.04.001
Fraser J, Steele N, Al Zaman A, Yule A (2011) Are patients in clinical trials representative of the general population? Dose intensity and toxicities associated with FE100C-D chemotherapy in a non-trial population of node positive breast cancer patients compared with PACS-01 trial group. Eur J Cancer 47:215–220. https://doi.org/10.1016/j.ejca.2010.10.001
Madarnas Y, Dent SF, Husain SF et al (2011) Real-world experience with adjuvant fec-d chemotherapy in four Ontario regional cancer centres. Curr Oncol 18:119–125
Oosting SF, van der Veldt AAM, GeurtsvanKessel CH et al (2021) mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol 22:1681–1691. https://doi.org/10.1016/S1470-2045(21)00574-X
Thakkar A, Gonzalez-Lugo JD, Goradia N et al (2021) Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell 39:1081-1090.e2. https://doi.org/10.1016/j.ccell.2021.06.002
Vogel M, Augusto G, Chang X et al (2022) Molecular definition of severe acute respiratory syndrome coronavirus 2 receptor-binding domain mutations: receptor affinity versus neutralization of receptor interaction. Allergy 77:143–149. https://doi.org/10.1111/all.15002
Fendler A, Shepherd STC, Au L et al (2021) Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: the CAPTURE study. Nat Cancer. https://doi.org/10.1038/s43018-021-00274-w
Buttiron Webber T, Provinciali N, Musso M et al (2021) Predictors of poor seroconversion and adverse events to SARS-CoV-2 mRNA BNT162b2 vaccine in cancer patients on active treatment. Eur J Cancer 159:105–112. https://doi.org/10.1016/j.ejca.2021.09.030
Zagouri F, Terpos E, Fiste O et al (2021) SARS-CoV-2 neutralizing antibodies after first vaccination dose in breast cancer patients receiving CDK4/6 inhibitors. Breast 60:58–61. https://doi.org/10.1016/j.breast.2021.08.017
Perry C, Luttwak E, Balaban R et al (2021) Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv 5:3053–3061. https://doi.org/10.1182/bloodadvances.2021005094
van Assen S, Holvast A, Benne CA et al (2010) Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum 62:75–81. https://doi.org/10.1002/art.25033
Eisenberg RA, Jawad AF, Boyer J et al (2013) Rituximab-treated patients have a poor response to influenza vaccination. J Clin Immunol 33:388–396. https://doi.org/10.1007/s10875-012-9813-x
Gelinck LBS, Teng YKO, Rimmelzwaan GF et al (2007) Poor serological responses upon influenza vaccination in patients with rheumatoid arthritis treated with rituximab. Ann Rheum Dis 66:1402–1403. https://doi.org/10.1136/ard.2007.071878
Gustafson CE, Jadhav R, Cao W et al (2020) Immune cell repertoires in breast cancer patients after adjuvant chemotherapy. JCI Insight 5(4):e134569. https://doi.org/10.1172/jci.insight.134569
Ingelman-Sundberg HM, Saghafian-Hedengren S, Jahnmatz M et al (2015) Selective loss of vaccine-specific memory B cells in a rhesus macaque model of chemotherapy: influence of doxorubicin on immunological memory. Haematologica 100:e158–e161. https://doi.org/10.3324/haematol.2014.116111
Kustin T, Harel N, Finkel U et al (2021) Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med 27:1379–1384. https://doi.org/10.1038/s41591-021-01413-7
Madhi SA, Baillie V, Cutland CL et al (2021) Efficacy of the ChAdOx1 nCoV-19 covid-19 vaccine against the B.1.351 variant. N Engl J Med 384:1885–1898. https://doi.org/10.1056/NEJMoa2102214
Mlcochova P, Kemp SA, Dhar MS et al (2021) SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 599:114–119. https://doi.org/10.1038/s41586-021-03944-y
Pulliam JRC, van Schalkwyk C, Govender N et al (2022) Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. Science. https://doi.org/10.1126/science.abn4947
Accorsi EK, Britton A, Fleming-Dutra KE et al (2022) Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. https://doi.org/10.1001/jama.2022.0470
Collie S, Champion J, Moultrie H et al (2022) Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med 386:494–496. https://doi.org/10.1056/NEJMc2119270
Pajon R, Doria-Rose NA, Shen X et al (2022) SARS-CoV-2 omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med 386:1088–1091. https://doi.org/10.1056/NEJMc2119912
Cancer. In: COVID-19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/special-populations/cancer/. Accessed 6 Dec 2021
Desai A, Gainor JF, Hegde A et al (2021) COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat Rev Clin Oncol 18:313–319. https://doi.org/10.1038/s41571-021-00487-z
Khoury DS, Cromer D, Reynaldi A et al (2021) Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 27:1205–1211. https://doi.org/10.1038/s41591-021-01377-8
Acknowledgements
We would like to thank all the patients for participating in this study. We are also grateful to Shinobu Makino (Department of Breast Surgery, Nagoya City University Graduate School of Medical Sciences) for her technical assistance, Junko Sugiyama (Department of Breast Surgery, Nagoya City University Graduate School of Medical Sciences) for her solid administrative support, and the Breast Surgical Trials of Clinical Oncology (B-STRO) members involved in this study. We also thank H. Nikki March, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This study was supported by an academic incentive donation (KIFUK20064).
Author information
Authors and Affiliations
Contributions
MT, NT, and TT: Contributed to the study conception and design; NK, YWE, TF, TA, TH, HS, KM, AW, ET, KS, RY, and TT: Contributed to the provision of study materials or patients; MT and AK: Contributed to the in vitro experimental procedures; MT, YU, AK, NY, AW, ET, KS, and RY: Collected and assembled the clinical data from medical records; MT, NT, and TT: Contributed to the data analysis and interpretation; MT wrote the manuscript. All authors finally approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The first protocol for this study (protocol no. 60–21-0019) was approved by the institutional review board of Nagoya City University Graduate School of Medical Sciences in June 2021. This study conformed to the guidelines of the Declaration of Helsinki. SARS-CoV-2 vaccinations started in Japan in February 2021 with Pfizer-BioNTech BNT162b2 mRNA vaccine (BNT162b2 vaccine). Three months later, mRNA-1273 Moderna COVID-19 vaccine (mRNA-1273 vaccine) was approved in Japan in March 2021. The interventional amendment to the protocol and subsequent enrollment for patients with mRNA-1273 was activated in July 2021.
Consent to participate
At enrollment, written informed consent was collected from all participants.
Consent to publish
All participants signed informed consent regarding publishing their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Terada, M., Kondo, N., Wanifuchi-Endo, Y. et al. Efficacy and impact of SARS-CoV-2 vaccination on cancer treatment for breast cancer patients: a multi-center prospective observational study. Breast Cancer Res Treat 195, 311–323 (2022). https://doi.org/10.1007/s10549-022-06693-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06693-2