Abstract
Background
The combination of a taxane with trastuzumab and pertuzumab is standard of care for first-line treatment of human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer. The combination of vinorelbine with trastuzumab and pertuzumab showed anti-tumor activity in a phase 2 trial.
Patients and methods
The databases of two tertiary medical centers were retrospectively searched for patients with HER2-positive metastatic breast cancer who underwent first-line treatment in 2013–2019 with a taxane or vinorelbine in combination with trastuzumab and pertuzumab. Groups were compared for progression-free survival (PFS), overall survival (OS), and toxicity profile.
Results
The study included 87 patients in the taxane group and 65 in the vinorelbine group. Overall median PFS was significantly longer in the taxane group [HR 0.56 (0.36–0.88), P = 0.01], but on multivariate analysis the difference was not statistically significant [HR 0.68 (0.4–1.1, P = 0.11)]. PFS was comparable in both groups of patients with recurrent disease [HR 0.94 (0.5–1.79), P = 0.85]. However, in patients with de novo metastatic disease, the difference in favor of the taxane group was pronounced [HR 0.4 (0.2–0.78), P = 0.007] and maintained significance on multivariate analysis [HR 0.46 (0.2–0.97, P = 0.04)]. There was no statistical significant difference in OS in the whole cohort [HR 0.69 (0.39–1.23)] or the subgroups.
Conclusions
Patients with HER2-positive metastatic breast cancer had similar survival with first-line treatment of taxane or vinorelbine combined with trastuzumab and pertuzumab. When the analysis was adjusted for prognostic factors, there was no PFS benefit for taxanes except in the subgroup with de novo disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common malignancy in females in the United States and the second most frequent cause of cancer death [1]. In 15–20% of cases, breast cancer overexpresses human epidermal growth factor receptor 2 (HER2) which results in more aggressive disease and historically in a poor prognosis [2, 3]. The treatment landscape for HER2-positive breast cancer has evolved considerably in the last three decades [4].
In 2015, the CLEOPATRA trial established the combination of docetaxel, trastuzumab and pertuzumab as the standard of care for first line treatment of HER2-positive metastatic breast cancer based on an improved overall survival compared to docetaxel, trastuzumab, and placebo [5, 6]. Subsequently, the PERUSE study compared docetaxel, paclitaxel or nab-paclitaxel combined with trastuzumab and pertuzumab. Preliminary results suggested that paclitaxel had similar efficacy as docetaxel [7] and could serve as a valid alternative in this setting. The HERNATA trial compared docetaxel plus trastuzumab with vinorelbine plus trastuzumab and showed comparable efficacy with a trend to a better outcome for estrogen-receptor-positive disease in the vinorelbine arm and with significantly fewer grade 3–4 adverse events [8].
The combination of vinorelbine, trastuzumab, and pertuzumab has shown efficacy and was well tolerated in the phase 2 VELVET trial [9], but the outcome of this combination compared to standard treatment with taxanes and anti-HER2 treatment has not been tested prospectively. The superior toxicity profile of vinorelbine as well as it’s comparable efficacy with paclitaxel combined with trastuzumab makes the combination of vinorelbine, trastuzumab, and pertuzumab a reasonable option as first-line therapy in certain cases. However, there are as yet no prospective or retrospective outcome studies comparing a taxane with vinorelbine combined with trastuzumab and pertuzumab.
The aim of the present retrospective two-center study was to evaluate the outcomes of patients with HER2-positive metastatic breast cancer treated with a taxane or vinorelbine combined with trastuzumab and pertuzumab as first line treatment.
Patients and methods
Study population
We searched the cancer center pharmacy database of two tertiary medical centers in Israel for all female patients aged 18 + years with HER2-positive advanced breast cancer who were treated with trastuzumab and pertuzumab combined with a taxane (paclitaxel, docetaxel or nab-paclitaxel) or with vinorelbine (intravenous or oral) in the first-line metastatic setting between 3/2013 and 9/2019. The data cutoff date was 1/2020. HER2-positive disease was defined as immunohistochemistry (IHC) result 3 + or HER2/chromosome enumeration probe 17 [CEP17] ratio ≥ 2 on fluorescence in situ hybridization. Patients who received other systemic therapy for metastatic disease prior to trastuzumab and pertuzumab administration were excluded. Patients who received trastuzumab plus pertuzumab combined with other chemotherapies were excluded. Patients included in the analysis had a minimum of 4 months of follow-up from the beginning of treatment.
Treatment protocols
All patients received intravenous pertuzumab 840 mg loading dose followed by 420 mg q3w, along with intravenous trastuzumab 8 mg/kg (loading dose) followed by 6 mg/kg q3w.
Treatment protocols in the taxane group included in this study were weekly paclitaxel (80 mg/m2), 3-weekly docetaxel (75 mg/m2), weekly docetaxel (35 mg/m2) and weekly nab-paclitaxel. Treatment protocols in the vinorelbine group were weekly intravenous vinorelbine (25 mg/m2) or weekly oral vinorelbine (60 mg/m2, with escalation to 80 mg/m2 according to tolerability).
Data collection
Demographic and clinical characteristics were recorded retrospectively from the electronic medical records, including age, performance status, visceral disease, previous treatments and stage at diagnosis. Response evaluation was based upon imaging reports using Response Evaluation Criteria in Solid Tumors (RECIST) criteria [9]. If imaging reports were not available or equivocal, then physicians’ notes summarizing the response evaluation were used.
Progression-free survival (PFS) was defined as time from treatment initiation to first evidence of radiographic progression or death from any cause. Overall survival (OS) was defined as time from treatment initiation to death from any cause. Data on toxicity were also collected including myelosuppression, diarrhea, nausea, peripheral neuropathy, asthenia and cardiotoxicity. Evaluation of toxicity was based upon National Cancer Institute Common Terminology Criteria for Adverse Events v5.0 grading [10].
Outcome measures
The primary endpoints of the study were PFS and OS. The secondary endpoint was toxicity. The study was approved by the Institutional Review Board of both institutions.
Statistical analysis
The statistical analysis was generated using SAS Software, version 9.4.
Continuous variables were presented by median (range), categorical variables were presented by (N, %).
T test was used to compare the value of continuous variables between study groups and Fisher’s exact test (for two groups) or Chi-square (for more than two groups) were used to compare the value of categorical variables between study groups.
PFS and OS were assessed by Kaplan–Meier survival analysis with the log-rank test. The Cox proportional hazards model was used to assess hazard ratios (HR) and 95% confidence intervals. Multivariate Cox proportional hazards regression was performed to explore the effect of treatment after adjusting for other known prognostic factors: Eastern Cooperative Oncology Group Performance Status (ECOG PS), age, visceral disease, de novo metastatic disease, and hormonal receptor status.
Two-sided P values less than 0.05 were considered statistically significant. For interaction tests P values less than 0.15 were considered significant.
Results
Study population and treatment patterns
We identified a total of 397 patients with HER2-positive breast cancer who were treated with trastuzumab and pertuzumab during the study period. Of these, 200 patients who were treated only in the adjuvant setting were excluded, as were 16 patients who received trastuzumab and pertuzumab as second-line treatment, 6 who were also given other cytotoxic drugs (capecitabine, cisplatin, 5-fluorouracil), and 5 who did not receive chemotherapy. An additional 18 patients did not have sufficient follow-up data for analysis (less than 4 months). The remaining 152 patients formed the final study cohort (see CONSORT chart, Fig. 1), of whom 87 received a taxane (78 paclitaxel, 9 docetaxel) and 65 received vinorelbine (49 intravenous, 16 oral). Four patients had initially received paclitaxel but had an allergic reaction after one dose and were switched to vinorelbine. These patients were included in the vinorelbine arm. No patients in our cohort received 3-weekly paclitaxel or nab-paclitaxel combined with trastuzumab and pertuzumab. Dose reduction was performed in 26% of patients in the taxane group and in 43% of patients in the vinorelbine group (P < 0.001). No patients received treatment as part of a clinical trial.
The patients’ baseline parameters are shown in Table 1. There were several significant between-group differences. The rate of metastasis at diagnosis was 67.8% in the taxane group and 41.5% in the vinorelbine group (P = 0.002). The respective rates of trastuzumab treatment in the adjuvant setting were 19.5% and 44.1% (P = 0.003), and of taxane treatment in the adjuvant setting, 23.5% and 48.3% (P = 0.003). More patients in the taxane group were asymptomatic (ECOG PS-0) at the onset of therapy (44.8% vs 24.6%, P = 0.04), and more patients in the vinorelbine group were over 70 years (26% vs 13%, P < 0.0001).
In 30 patients with ER positive disease (22 in the taxane group, 8 in the vinorelbine group, P < 0.001) who achieved clinical benefit, chemotherapy was withheld and maintenance hormonal therapy was given in combination with trastuzumab and pertuzumab. The median duration of chemotherapy was 22 weeks and 32 weeks, for taxane and vinorelbine, respectively (P = 0.008). Dual anti-her2 inhibition as maintenance therapy was administered to 76% of patients in the taxane group for a median of 80 weeks and to 48% of the vinorelbine group for a median of 38 weeks (P < 0.0001). Median duration of follow-up was 44 months (IQR 28–65 months) and 23 months (IQR 14–31 months) in the taxane and vinorelbine groups, respectably.
Median duration from surgery in the early stage to initiation of treatment in relapsed metastatic disease was 54 months in the taxane group and 45 months in the vinorelbine group. No patients received trastuzumab or pertuzumab in the 6 months preceding initiation of treatment in the metastatic setting.
Progression-free survival
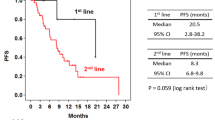
Median PFS was 32.9 months in the taxane group and 14.2 months in the vinorelbine group; the difference was statistically significant [HR 0.56 (0.36–0.88), P = 0.01] (Fig. 2a). On multivariate Cox proportional hazards regression adjusted for age, ECOG PS, de novo metastatic disease status, ER status, and visceral disease, there was no significant between-group difference in PFS [HR 0.68 (0.4–1.1), P = 0.11].
In the subgroup of patients with recurrent disease, there was no significant difference in PFS between those treated with a taxane or vinorelbine; median PFS was 19.6 months and 14.3 months, respectively [HR 0.94 (0.51–1.75), P = 0.85] (Fig. 2b). However, in the subgroup of patients with de novo disease, the difference in PFS was pronounced: 35.5 months in the taxane group and 14.2 months in the vinorelbine group [HR 0.4 (0.2–0.78), P = 0.007] (Fig. 2c). The test of interaction for the effect of chemotherapy between the de-novo and recurrent sub-groups was significant with a P value of 0.09. On multivariate Cox proportional hazards regression adjusted for type of therapy, age, ECOG PS, ER status, and visceral disease, taxane therapy was significantly associated with an improved PFS [HR 0.46 (0.2–0.97), P = 0.04].
In the subgroup of patients with no visceral disease, median PFS was 33 months in the taxane group and 14.2 months in the vinorelbine group [HR 0.46 (0.23–0.91), P = 0.03)]. On multivariate Cox proportional hazards regression adjusted for type of therapy, age, ECOG PS, ER status, and de novo disease status, the advantage of taxane therapy lost statistical significance [HR 0.54 (0.24–1.19), P = 0.13]. In patients with visceral disease, the difference in PFS was not significant, with median values of 28.5 months and 15.5 months in the taxane and vinorelbine groups, respectively [HR 0.69 (0.38–1.25), P = 0.22].
Overall survival
The difference in median OS between the taxane and vinorelbine groups did not reach statistical significance on analysis of the whole cohort [56 months vs 41 months; HR 0.69 (0.39–1.23), P = 0.2] (Fig. 3a). Nor did it reach statistical significance in the subgroups of patients with recurrent disease [30.8 months vs 38.9 months; HR 1.3 (0.61–2.87), P = 0.48] (Fig. 3b) or de novo metastatic disease [59.5 months vs 41.3 months; [HR 0.43 (0.17–1.04), P = 0.055] (Fig. 3c), despite an 18-month advantage for the taxane group in de novo metastatic disease. In the subgroups of patients with and without visceral disease no significant statistical difference was reached [visceral disease: 45.23 months vs 41.03 months, HR 0.95 (0.44–2.02), P = 0.88; no visceral disease: 59.6 months vs 43.6 months, HR 0.53 (0.22–1.28), P = 0.15].
Toxicity
Toxicity led to a cessation of therapy in 40% of patients in the taxane group compared to 22.4% in the vinorelbine group (P = 0.04). More patients in the vinorelbine than the taxane group had neutropenia which resulted in a dose reduction (17% vs 1%, P < 0.001). While more patients in the taxane group experienced peripheral neuropathy (34.5% vs 4.6%, P < 0.001). Cardiotoxicity defined as a decline of 10% in left ventricular ejection fraction or symptomatic left ventricular dysfunction was more common in patients treated with a taxane than those treated with vinorelbine (6.9% vs 4.6%, P = 0.014). Symptomatic left ventricular dysfunction was experienced in 2 patients (2.3%) in the taxane group compared to 1 patient in the vinorelbine group (1.5%).
Discussion
This is the first study to compare dual blockade (with trastuzumab and pertuzumab) combined with a taxane or with vinorelbine as first-line treatment in patients with HER2-positive metastatic breast cancer. While combination with a taxane is considered standard of care in this setting [4, 5], it has considerable toxicity which can have a profound adverse impact on quality of life [11, 12].
We did not find a statistically significant OS advantage for vinorelbine or taxane, either in the whole cohort or the various subgroups. However, a numerical difference of 15 months favoring the taxane arm was observed. It is noteworthy that among patients with de novo disease, the survival advantage for taxanes was pronounced (18.2 months), though it did not reach statistical significance (P = 0.055). Regarding PFS, when the analysis was adjusted for prognostic factors, compared to vinorelbine, taxane therapy was associated with significantly longer PFS for de novo metastatic disease, both on univariate analysis and multivariate analysis, with an absolute difference of 21.3 months. No advantage was observed for taxanes in patients with recurrent disease.
Sixteen patients in our cohort received oral vinorelbine. The bioequivalence of oral and intravenous vinorelbine was demonstrated in several pharmacokinetic studies [13,14,15]. The clinical efficacy of oral vinorelbine was demonstrated in several prospective clinical trials as single agent and in combinations [16]. Based on these studies, oral and intravenous vinorelbine were, therefore, grouped together in the analyses.
There were several meaningful differences in background parameters between the treatment groups which could limit the interpretation of the results. Patients who received first-line therapy with vinorelbine were more likely to be older than 70 years, have a worse ECOG PS, and have recurrent disease (44% after being treated with trastuzumab and 48% with a taxane in the adjuvant setting) and have visceral disease. Nevertheless, our results indicate that at least for recurrent disease, vinorelbine could serve as the chemotherapy component of the treatment protocol.
The most recent prospective study of the efficacy and safety of vinorelbine combined with trastuzumab and pertuzumab was the phase 2 VELVET trial [17, 18]. This trial was a non-randomized prospective phase 2 trial with two cohorts—in cohort 1 trastuzumab and pertuzumab were administered in separate infusions, whereas in cohort 2 the two antibodies were given in a single infusion bag. Although outcome results were satisfactory with predicted toxicity, median PFS (14.3 months and 11.5 months in cohort 1 and 2, respectively) and duration of response were shorter than when a taxane was combined with trastuzumab and pertuzumab in the CLEOPATRA and PERUSE trials (18.1 and 20.6 months, respectively) [5,6,7]. However, caution is needed when making cross-trial comparisons owing to the different study designs and patient populations, including a lower proportion of patients with de novo metastatic disease, a higher frequency of visceral disease at baseline and a higher percentage of patients who received neoadjuvant or adjuvant trastuzumab in the VELVET trials compared to the CLEOPATRA trial.
A retrospective population-based trial from Denmark examined first-line treatment patterns and outcomes in 291 patients with HER2-positive metastatic breast cancer in a real-world setting. Trastuzumab and pertuzumab were combined with vinorelbine in 81% of patients and with a taxane in 12%. Median PFS was 15.8 months for the whole population and 17.9 months for the 112 patients with de novo metastatic disease. Among the patients with recurrent disease, median PFS was 16.5 months for those who received trastuzumab as adjuvant treatment and 15.0 months for those who did not [19]. Median OS was 41.8 months for the whole cohort and was not reached for the patients with de novo metastatic disease. Among the patients with recurrent disease, median OS was 41.3 months for those who received adjuvant trastuzumab and 35.6 months for those who did not. The authors attributed the poorer survival in this study compared to the CLEOPATRA trial [5, 6] to the older age of the patients, lower proportion of patients with de novo metastatic breast cancer, and shorter time on dual blockade (11.1 months vs 17.4 months). Inferior efficacy of vinorelbine relative to docetaxel could not be excluded. When compared to the current study—the median overall survival of 41 months was similar to our vinorelbine group. No comparison was conducted between taxane and vinorelbine therapy in this study. Several real-world studies from Italy and the USA reporting experience with dual HER2 inhibition have been published. Most patients in these trials were treated with a taxane. Median PFS in these trials was 16.9–27.8 months [20,21,22].
The median PFS of 32.9 months achieved in the taxane group in the present study compares favorably with the 18.7 months in the CLEOPATRA trial [5, 6] and 20.6 months in the PERUSE trial (19.6, 23.0 and 18.1 months with docetaxel, paclitaxel, and nab-paclitaxel, respectively) [7]. Additionally, the median OS of 56 months in our taxane group is similar to that of the docetaxel arm in the CLEOPATRA trial (56.5 months). In our vinorelbine group, the median PFS of 14.1 months is close to that reported in the VELVET trial (14.3 months in cohort 1, 11.5 months in cohort 2) [17, 18]. The VELVET trial did not report OS data. Comparison of the median OS with the CLEOPATRA trial yielded a lower value in our vinorelbine group (41 months), but the difference may be due to the different patient populations.
In the present study, patients with de novo metastatic disease had a better prognosis than patients with recurrent disease in terms of both PFS [HR 0.6 (0.38–0.99)] and OS [HR 0.45 (0.24–0.83)]. A recent observational study reported similar results [23], suggesting that these two groups have different disease characteristics and outcomes.
The main limitation of our study is it’s retrospective design which has inherent biases, including a prescribing bias and between-group differences in prognostic parameters (significantly less favorable in the vinorelbine group). However, multivariate analysis was performed to address some of these limitations. A larger cohort might have exposed still more between-group differences. Another limitation is the relatively small sample size, and therefore, some of the subgroup analyses should be interpreted with caution. As patients were treated only in two centers there could potentially be biases regarding local clinical practice. However, we do believe that practice in our institutions represents the accepted standard of care following international guidelines. The strengths of the study are the combined data from two tertiary institutes and the completeness of the data for the patients included in the analyses.
Given the difference in toxicity profile between taxanes and vinorelbine, which significantly favored vinorelbine, and the potential impact on survival of second- and third-line treatments, more studies challenging the standard of care are required. Ideally, a randomized trial should be conducted to evaluate the role of vinorelbine as first-line therapy for HER2-positive metastatic breast cancer in patients with recurrent disease.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34. https://doi.org/10.3322/caac.21551
Howlader N, Altekruse SF, Li CI et al (2014) US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. https://doi.org/10.1093/jnci/dju055
Ross JS, Slodkowska EA, Symmans WF et al (2009) The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 14:320–368. https://doi.org/10.1634/theoncologist.2008-0230
Gradishar WJ, Abraham J, Aft R et al (2019) NCCN Guidelines Version 1.2019 Breast Cancer NCCN Guidelines
Swain SM, Baselga J, Kim S-B et al (2015) Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372:724–734. https://doi.org/10.1056/NEJMoa1413513
Baselga J, Cortés J, Kim S-B et al (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. https://doi.org/10.1056/NEJMoa1113216
Bachelot T, Ciruelos E, Schneeweiss A et al (2019) Preliminary safety and efficacy of first-line pertuzumab combined with trastuzumab and taxane therapy for HER2-positive locally recurrent or metastatic breast cancer (PERUSE). Ann Oncol 30:766–773. https://doi.org/10.1093/annonc/mdz061
Andersson M, Lidbrink E, Bjerre K et al (2011) Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2–positive breast cancer: the HERNATA study. J Clin Oncol 29:264–271. https://doi.org/10.1200/JCO.2010.30.8213
Eisenhauer EA, Therasse P, Bogaerts J, et al (2008) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). https://doi.org/10.1016/j.ejca.2008.10.026
Cancer Institute N (2017) Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0
Shimozuma K, Ohashi Y, Takeuchi A et al (2012) Taxane-induced peripheral neuropathy and health-related quality of life in postoperative breast cancer patients undergoing adjuvant chemotherapy: N-SAS BC 02, a randomized clinical trial. Support Care Cancer 20:3355–3364. https://doi.org/10.1007/s00520-012-1492-x
Rivera E, Cianfrocca M (2015) Overview of neuropathy associated with taxanes for the treatment of metastatic breast cancer. Cancer Chemother Pharmacol 75:659–670. https://doi.org/10.1007/s00280-014-2607-5
Marty M, Fumoleau P, Adenis A et al (2001) Oral vinorelbine pharmacokinetics and absolute bioavailability study in patients with solid tumors. Ann Oncol 12:1643–1649. https://doi.org/10.1023/A:1013180903805
Bourgeois H, Vermorken J, Dark G et al (2007) Evaluation of oral versus intravenous dose of vinorelbine to achieve equivalent blood exposures in patients with solid tumours. Cancer Chemother Pharmacol 60:407–413. https://doi.org/10.1007/s00280-007-0510-z
Variol P, Nguyen L, Tranchand B, Puozzo C (2002) A simultaneous oral/intravenous population pharmacokinetic model for vinorelbine. Eur J Clin Pharmacol 58:467–476. https://doi.org/10.1007/s00228-002-0506-x
Aapro M, Finek J (2012) Oral vinorelbine in metastatic breast cancer: a review of current clinical trial results. Cancer Treat Rev 38:120–126
Perez EA, López-Vega JM, Petit T et al (2016) Safety and efficacy of vinorelbine in combination with pertuzumab and trastuzumab for first-line treatment of patients with HER2-positive locally advanced or metastatic breast cancer: VELVET Cohort 1 final results. Breast Cancer Res 18:126. https://doi.org/10.1186/s13058-016-0773-6
Andersson M, López-Vega JM, Petit T et al (2017) Efficacy and safety of pertuzumab and trastuzumab administered in a single infusion bag, followed by vinorelbine: VELVET cohort 2 final results. Oncologist 22:1160–1168. https://doi.org/10.1634/theoncologist.2017-0079
Christensen T, Berg T, Nielsen LB et al (2020) Dual HER2 blockade in the first-line treatment of metastatic breast cancer—a retrospective population-based observational study in Danish patients. Breast 51:34–39. https://doi.org/10.1016/j.breast.2020.03.002
Robert NJ, Goertz H-P, Chopra P et al (2017) HER2-positive metastatic breast cancer patients receiving pertuzumab in a community oncology practice setting: treatment patterns and outcomes. Drugs Real World Outcomes 4:1–7. https://doi.org/10.1007/s40801-016-0102-5
De Placido S, Giuliano M, Schettini F et al (2018) Human epidermal growth factor receptor 2 dual blockade with trastuzumab and pertuzumab in real life: Italian clinical practice versus the CLEOPATRA trial results. Breast 38:86–91. https://doi.org/10.1016/j.breast.2017.12.012
Gamucci T, Pizzuti L, Natoli C et al (2019) A multicenter REtrospective observational study of first-line treatment with PERtuzumab, trastuzumab and taxanes for advanced HER2 positive breast cancer patients. RePer Study Cancer Biol Ther 20:192–200. https://doi.org/10.1080/15384047.2018.1523095
Tripathy D, Brufsky A, Cobleigh M et al (2020) De novo versus recurrent HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from the SystHERs registry. Oncologist 25:e214–e222. https://doi.org/10.1634/theoncologist.2019-0446
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
DR: conceptualization, methodology, data curation, formal analysis, investigation, validation, writing–original draft, and writing–review and editing, final approval of the version to be submitted; IK: conceptualization, methodology, data curation, formal analysis, investigation, validation, writing–original draft, and writing–review and editing, final approval of the version to be submitted; TS: formal analysis, writing–review and editing, final approval of the version to be submitted; BN: validation, writing–review and editing, final approval of the version to be submitted; AS: writing–original draft, and writing–review and editing, final approval of the version to be submitted; DH: investigation, writing–review and editing, final approval of the version to be submitted; OR: investigation, writing–review and editing, final approval of the version to be submitted; DT: investigation, writing–review and editing, final approval of the version to be submitted. OO: investigation, validation, writing–review and editing, final approval of the version to be submitted; HG: investigation, validation, writing–review and editing, final approval of the version to be submitted. MS: investigation, writing–review and editing, final approval of the version to be submitted. VN: investigation, writing–review and editing, final approval of the version to be submitted. JP: investigation, validation, data curation, writing–review and editing, final approval of the version to be submitted. MG: investigation, writing–review and editing, final approval of the version to be submitted; SY-K: investigation, writing–review and editing, final approval of the version to be submitted; RY: conceptualization, methodology, formal analysis, validation, writing–original draft, and writing–review and editing, final approval of the version to be submitted.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Kuchuk declared honorarium payment from Roche, Pfizer, Novartis, Eli Lilly, all outside the submitted manuscript. Dr. Goldvaser declared honorarium payment from Roche, Pfizer, Novartis, Oncotest, all outside the submitted manuscript. Dr. Yust-Katz declared honorarium payment from Teva, Astra-Zeneca, Novartis and receiving a research grant from BMS. Dr. Yerushalmi reports personal fees from Roche (Consulting, Invited speaker), Pfizer (Consulting), Novartis (Consulting), Teva (Invited speaker), Medison (Invited speaker), Dexel (Consulting), MSD (Invited speaker), Astra-Zeneca (Invited speaker) and Novartis (Invited speaker), all outside the submitted manuscript. The other authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reinhorn, D., Kuchuk, I., Shochat, T. et al. Taxane versus vinorelbine in combination with trastuzumab and pertuzumab for first-line treatment of metastatic HER2-positive breast cancer: a retrospective two-center study. Breast Cancer Res Treat 188, 379–387 (2021). https://doi.org/10.1007/s10549-021-06198-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06198-4