Abstract
Purpose
Patients with ErbB2/Her2 oncoprotein-positive breast cancers often receive neoadjuvant therapies (NATs) containing the anti-ErbB2 antibody trastuzumab. Tumors that are still present after NATs are resected, and patients continue receiving trastuzumab. These cancers are associated with high relapse risk. Whether relapse will occur cannot be presently reliably predicted. The ability to make such predictions could improve disease management. We found previously that ErbB2 blocks breast tumor cell anoikis, apoptosis induced by cell detachment from the extracellular matrix, by downregulating the pro-apoptotic protein Irf6 and upregulating the anti-apoptotic protein Epidermal Growth Factor Receptor (EGFR) in the cells and, thus, promotes their three-dimensional growth. We now tested whether tumor levels of these proteins before and after NATs correlate with patients’ relapse-free survival (RFS) and overall survival (OS).
Methods
We selected archival breast tumor samples collected from 37 women with ErbB2-positive stages II and III breast cancer before and after NATs. We used immunohistochemistry to test whether levels of the indicated proteins in respective tumors correlate with RFS and OS.
Results
We observed that the presence of high Irf6 levels in the tumors following NATs correlated with reduced RFS and OS. Perhaps not by coincidence, we noticed that trastuzumab-sensitive ErbB2-positive breast cancer cells selected for the ability to overproduce exogenous Irf6 in culture acquired trastuzumab resistance. Finally, EGFR presence in patients’ tumors before or after NATs was associated with decreased RFS and OS.
Conclusions
This study could help identify patients with ErbB2-positive tumors that are at increased risk of disease relapse following NATs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
15–20% of breast tumors overproduce ErbB2 (ErbB2 Receptor Tyrosine Kinase 2)/Her2 (human epidermal growth factor receptor 2) receptor tyrosine kinase which drives these cancers [1]. Anti-ErbB2 antibody trastuzumab [2] is used for treatment of ErbB2-positive malignancies.
Patients with ErbB2-positive breast cancer often receive neoadjuvant trastuzumab and chemotherapy for 12–18 weeks [3]. Such therapy causes complete tumor regression in approximately 30% of patients, while the rest of the patients display partial tumor regression or stable disease [4]. Patients whose tumors are still present after trastuzumab-based neoadjuvant therapies undergo surgical tumor resection and continue receiving the therapies to complete a 1-year course [3]. Cancer relapses in about 30% patients [3]. Whether the relapse will occur cannot be presently reliably predicted [5]. Trastuzumab can cause serious side effects, e.g., cardiotoxicity [6], and is costly [7]. Thus, identifying patients who are at increased risk of disease relapse could allow oncologists to spare these patients the side effects of unwarranted therapy and/or modify the treatments to improve disease outcomes. We identified recently several critical mediators of ErbB2-driven breast cancer [8, 9] and tested in this study whether the levels of these mediators in the tumors before and after neoadjuvant trastuzumab-based therapies predict disease relapse.
One essential feature of primary and disseminated breast tumors, including those overproducing ErbB2, is their ability to grow in a three-dimensional manner [10]. Such growth requires the ability of cancer cells to survive without adhesion to the extracellular matrix (ECM) [11]. This notion is based on the fact that normal breast epithelial cells are attached to the ECM in the breast [12, 13], and detachment causes their death by apoptosis [14], a phenomenon called anoikis [15]. In contrast, breast tumors grow and metastasize as three-dimensional cellular masses in which the cells are not properly attached to the ECM but remain viable [14]. Numerous data indicate that anoikis resistance of tumor cells, including that of breast cancer cells, is critical for tumor progression [14]. First, cancer cells survive and grow without adhesion to the ECM as colonies in soft agar. This ability is a “gold standard” for malignant transformation [16]. Second, major oncoproteins, e.g., ErbB2 [8] and Ras [17], block tumor cell anoikis. Finally, approaches that cause anoikis of tumor cells suppress their ability to form primary tumors [17,18,19,20] and metastases [18, 20,21,22]. As ErbB2 blocks breast tumor cells anoikis [9], mediators of this resistance are potential predictors of breast tumor response to ErbB2 antagonists.
We found that anoikis of non-malignant human breast epithelial cells occurs due to detachment-induced upregulation of Perp (p53 apoptosis effector related to PMP-22), an inducer of apoptosis [9]. Perp protein shows homology to members of the tetraspan family of proteins that control cell growth [23] and is an element of the desmosomes, multiprotein complexes mediating cell-to-cell adhesion [24]. Perp causes apoptosis by unknown mechanisms [25], and Perp loss in breast epithelium promotes breast carcinoma in mice [26]. We have observed that ErbB2 blocks anoikis of breast cancer cells by preventing detachment-induced Perp upregulation, and that the effect of ErbB2 on Perp is driven by ErbB2-dependent mitogen-activated protein kinase (MAPK)-mediated upregulation of epidermal growth factor receptor (EGFR) in cancer cells [9].
We further found that detachment of non-malignant mammary epithelial cells from the ECM upregulates transcription factor Irf6 (Interferon Regulatory Factor 6) [8]. Irf6, a member of the Irf transcription factor family [27], kills cells by apoptosis [28] and is likely an important mediator of breast function as Irf6 is upregulated in the breast during mammary gland regression upon cessation of lactation [29] (such regression is thought to involve anoikis of breast epithelial cells [30]). We observed that another ErbB2-dependent anoikis mechanism is driven by ErbB2-dependent MAPK-mediated downregulation of Irf6 in breast cancer cells [8]. Whether or not Irf6 and Perp are elements of two different ErbB2-dependent signaling pathways or those of the same ErbB2-driven pathway is presently not known.
Since Irf6, EGFR, and Perp represent critical mediators of ErbB2-driven mechanisms of breast cancer progression discovered by us [8, 9], we reasoned that changes in the levels of these proteins in breast tumor samples during trastuzumab-based breast cancer treatments could predict patients’ trastuzumab response. Thus, we investigated whether the levels of these proteins in patients’ tumors before and after neoadjuvant trastuzumab-based treatments or the changes in these levels following such treatments can predict cancer relapse in patients that received these therapies. We have established that high Irf6 levels in the tumors following the treatments, high EGFR levels in the tumors before or after the indicated therapies, or EGFR upregulation in the tumors following the treatments significantly correlates with disease relapse.
Patients and methods
All analyses followed recommendations for tumor marker prognostic studies [31].
Clinical studies
Upon research ethics board approval from the local health authorities, a list of patients with ErbB2-positive primary invasive breast cancers who underwent neoadjuvant chemotherapy and ErbB2-targeted therapy was obtained from the institutions’ pharmacy information system (BDM Pharmacy; BDM IT Solutions, Saskatoon, SK, Canada) and laboratory information systems (Cerner Millennium; Cerner, North Kansas City, MO, USA and Meditech, Westwood, MA, USA). Formalin-fixed paraffin-embedded (FFPE) archival breast tumor biopsies collected from women with clinical stages II and III at the time of diagnosis and FFPE tumor samples obtained from these patients during definitive surgery after neoadjuvant treatments (chemotherapy with or, in one case, without trastuzumab) were collected at QEII Health Centre, Halifax, NS (16 patients), Saint John Regional Hospital, Saint John, NB (11 patients) and the Moncton Hospital, Moncton, NB (10 patients).
Pathological features of patients’ tumors
Pathological features were derived from the initial diagnostic biopsy and the post-neoadjuvant excisional specimens. Hormonal receptor (ER/PR) and ErbB2 status were determined on the diagnostic biopsy specimen. Nottingham tumor grade, tumor type, and lymph node status were determined on the excisional specimens. The residual cancer burden was calculated based on the excisional specimens.
Patients’ clinical characteristics
Clinical data and chemotherapy regimens were abstracted from the patient electronic medical records. Date and cause of death were obtained from respective cancer registries and from the electronic medical records. Clinical stage was determined by clinical TNM stage at time of diagnosis.
Laboratory studies
Immunohistochemistry
Representative tissue blocks from the diagnostic core biopsies and post-neoadjuvant excisional specimens were selected for immunohistochemistry (IHC) after review of the H&E—as well as anti-ErbB2-stained IHC slides to confirm diagnosis and ErbB2 positivity. FFPE core biopsies and excisional specimens underwent heat-induced epitope retrieval for 24 min for Irf6 in Cell Conditioning 1 (Ventana Medical Systems, Tucson, AZ, USA) followed by 32-min incubation in 1:100 dilution of Irf6 antibody (MyBioSource, San Diego, CA, USA) or 1:200 dilution of EGFR antibody (Cell Marque, Rocklin, CA, USA) or 1:1500 dilution of primary Perp antibody (Thermo Fisher Scientific, Waltham, MA, USA). The reaction was detected using the OptiView polymer detection system on a Ventana Benchmark Ultra platform (Ventana Medical Systems). The staining protocol was validated according to the manufacturer’s instructions using human kidney specimens in the case of Irf6, human skin, breast epithelium, and colonic epithelium in the case of EGFR and human heart and skin specimens in the case of Perp. Cells were also counterstained with hematoxylin. Irf6 IHC data for the pre- and post-neoadjuvant trastuzumab-based therapies in the case of 10 patients whose samples were collected at the QEII Health Centre were previously published by us [8].
Cell positivity for Irf6, EGFR, and Perp was scored on the pre-neoadjuvant core biopsies and post-neoadjuvant excisional specimens following IHC by manually counting positively stained cells as a percentage of all breast cancer cells in the tumor sample.
Cell culture-based experiments
BT474 (American Type Culture Collection) and BT474TR [8] cells were cultured in Hybri-Care medium (American Type Culture Collection), 10% FBS, 100 U/ml penicillin (Thermo Fisher Scientific, Waltham, MA, USA), 100 μg/ml streptomycin (Thermo Fisher Scientific), and 0.29 mg/ml L-glutamine (Thermo Fisher Scientific). To detach the cells from the ECM, they were placed in suspension culture above a layer of 1% sea plaque agarose polymerized in the culture medium not containing any additional ingredients. To generate BT474control and BT474Irf6 cells, 293 T cells (2 × 106) were incubated with 5 μg of either control pBabehygro expression vector or pBabehygro-HA-Irf6 expression vector and 2.5 μg of pHIT and 2.5 μg of pVSVG expression vectors encoding retroviral proteins in the presence of 20 μl of Lipofectamine 2000 reagent in 6 ml of Opti-MEM medium. Generation of the pBabehygro-HA-Irf6 expression vector is described in [8]. The medium was changed 4 h later to DMEM containing 10% FBS. The medium was collected 48 h later and filtered through a 0.45-μm filter unit. The viral supernatant containing either the control virus or that encoding Irf6 was added to 2.5 × 105 BT474 cells grown on a 60-mm dish in the presence of 8 μg/ml polybrene in the presence of 300 μg/ml hygromycin for 48 h. The cells were then harvested, expanded, and used for the assays described in this study. Western blotting analysis of the cells was performed as described previously [32]. Anti-Irf6 and anti-GAPDH antibodies used for western blotting were from Cell Signaling Technology, Danvers, MA, USA. To assay the cells for trastuzumab sensitivity, the cells were grown in suspension as described above and counted.
Statistical analysis
Descriptive statistics were reported as counts and percentages for categorical variables, means (standard deviation SD) for normally distributed continuous variables, and medians (interquartile ranges IQR) for non-normally distributed continuous variables. Baseline differences between patients with and without relapse were examined using Chi-square test, Fisher’s exact test, T-test, and/or Wilcoxon rank-sum test, as appropriate. Receiver-operating curves were plotted for each of the proteins studied. An exploratory analysis was also performed to examine an optimal cut-point for each protein in question. A flexible graphical tool generated using a SAS macro was used to visualize the impact of changing the cut-points for the levels of the indicated proteins. The optimal cut-point in terms of sensitivity and specificity was selected to generate a dichotomized variable to be used in predictive screening for relapse-free survival, defined as the time from diagnosis to first relapse. Patients were censored on death or date of last follow-up. Rates of relapse were estimated by the Kaplan–Meier method and compared between the categorical groups for each of the indicated proteins and pre-identified baseline demographics including stage, grade of tumor, and lymph node status. The log-rank test was used to compare outcomes between the two groups. Cox proportional-hazards models were used to estimate hazard ratios. Plots of Martingale residuals were used to test the functional form of continuous variables. All analyses were conducted using SAS STAT 14.3 software version 9.4 (SAS Institute, Cary, N.C.), and a significance level of α = 0.05.
Results
Patients’ clinical characteristics and responses to trastuzumab-based neoadjuvant therapies
To study the levels of Irf6, Perp, and EGFR, the three critical mediators of ErbB2-dependent breast cancer cell anoikis resistance [8, 9], in stage II and III ErbB2-positive breast tumors before and after neoadjuvant therapies, we selected FFPE archival breast tumor biopsies collected from 37 women with the indicated form of breast cancer at the time of diagnosis before neoadjuvant therapies. Patients received neoadjuvant trastuzumab together with various combinations of chemotherapeutic drugs, e.g., 5-fluorouracil, epirubicin, cyclophosphamide, and docetaxel (FEC-D) or docetaxel and carboplatin or paclitaxel alone, whereas one patient received neoadjuvant FEC-D alone. Several patients received neoadjuvant pertuzumab in addition to trastuzumab. We also selected FFPE tumor samples obtained from these patients during definitive surgery after the therapies. All patients received adjuvant trastuzumab after tumor resection. Patients’ clinical and pathological characteristics are shown in Table 1.
High Irf6 levels in the tumors after neoadjuvant therapies are associated with disease relapse
Since ErbB2-driven downregulation of the pro-apoptotic transcription factor Irf6 blocks breast cancer cell anoikis [8], we performed IHC analysis of Irf6 levels in the breast tumor samples collected before and after neoadjuvant treatments. We validated IRF6 IHC using human kidney glomeruli as a positive control as per the ant-Irf6 antibody manufacturer’s instructions (see Patients and Methods) (Supplementary Fig. 1a) and human cardiac myocytes and germinal center cells from human tonsils and lymph nodes as negative controls [33] (Supplementary Fig. 1b, c). In addition, similar to what was published by others [33], our IHC conditions allowed us to detect Irf6 in benign breast ductal epithelium (cytoplasmic and nuclear), small intestinal villous epithelium (cytoplasmic), colonic columnar epithelium (cytoplasmic), placental trophoblasts (cytoplasmic and nuclear), and liver ductal cells (cytoplasmic) (Supplementary Fig. 1d–g). As we published [8], Irf6 displayed nuclear localization in the tumor cells in the majority of breast cancer samples that we used which is not surprising since being a transcription factor, Irf6 functions in the cell nuclei [34]. We observed that the percentage of Irf6-positive cells is increased approximately sixfold following neoadjuvant treatments (on average, from 0.9 to 5.4%) in 55.5% cases and is decreased approximately 11-fold (on average, from 2.1 to 0.2%) in 18.5% cases following these therapies (Fig. 1a–c). The indicated changes were statistically significant in both cases (Fig. 1a). The percentage of Irf6-positve cells was similar in the tumor samples before and after the treatments in 26% of patients (Fig. 1a, d). Neither the increase nor the decrease in the percentage of Irf6-positive cells following the treatments correlated significantly with patients’ relapse-free survival (not shown). A significant fraction (11%) of the tumors before the treatment displayed a relatively high percentage (more than 6%) of Irf6-positive cells (Fig. 2a). The presence of such Irf6 levels in the pre-treated tumors did not correlate with relapse-free survival (not shown). We further observed that 22% of the post-treated tumors displayed more than 6% of Irf6-positive cells (Fig. 2a, b). These levels ranged from 6 to 12%. Remarkably, the presence of these relatively high Irf6 levels in the post-treated tumors was significantly associated with reduced relapse-free survival: p value = 0.005, hazard ratio (HR) = 5.2, (95% confidence interval (CI) 1.4–19.1) (Fig. 2c). We further tested whether Irf6 levels in the post-neoadjuvant therapy tumor samples correlate with disease relapse when these levels are used as a continuous variable. We found that the HR of relapse increases by 17.2% per increase of the percentage of Irf6-positive cells in the indicated samples by each percent (from 0 to 12) (p value = 0.0270) with the initial HR being 1.172 (95% CI 1.018, 1.350) (Fig. 2d). In summary, relatively high Irf6 levels in the tumors after the neoadjuvant therapies are associated with higher risk of disease relapse.
Neoadjuvant therapy-dependent changes in Irf6 levels in patients’ tumors before and after the therapies. a Percentage of Irf6-positve cells in patients’ tumor samples collected before and after the therapies. b–d Representative samples obtained from patients before (left) and after (right) the treatments are shown. The samples were stained with the anti-Irf6 antibody (brown) and counterstained with hematoxylin (blue). Examples of an increase (b), decrease (c), or lack of change (d) in the Irf6 levels are shown
High Irf6 levels in patients’ tumors following neoadjuvant therapies are associated with disease relapse. a Percentage of patients with > 6% Irf6-positve cells in the tumor samples collected before and after the therapies. b A representative sample with > 6% Irf6-positve cells obtained from a patient after the treatment is shown. The sample was stained with the anti-Irf6 antibody (brown) and counterstained with hematoxylin (blue). c Kaplan–Meier analysis-based estimation of probabilities of patients’ relapse-free survival depending on whether % of Irf6-positve cells in the tumor samples collected after the therapies is higher or lower than 6%. d The results of analysis of the changes of HR of relapse in the case of continuous increase (from 0 to 12%) in % of Irf6-postitve cells in the samples collected after the therapies
Increased Irf6 expression in cultured ErbB2-positive breast cancer cells is associated with trastuzumab resistance
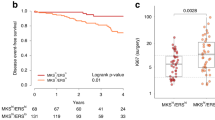
We observed previously that ErbB2 blocks cancer cell anoikis by downregulating Irf6 in the tumor cells, and that enforced Irf6 upregulation kills these cells [8], yet our current data indicate that relatively high Irf6 levels in breast tumor cells correlate with disease relapse. One possible explanation for these results is that during tumor progression, breast cancer cells acquire the signals rendering them resistant to trastuzumab-based therapies and that these signals allow the cells to overproduce Irf6 and survive in the presence of high Irf6 levels. The indicated signals could arise from genetic or epigenetic changes occurring in the cells. Alternatively, it is possible that in the course of disease progression, tumor cells acquire mechanisms protecting them from Irf6-dependent death. Conceivably, the signals that render the cells resistant to the presence of high Irf6 levels also make them resistant to trastuzumab-based therapies. Consequently, the presence of relatively high Irf6 levels in cancer cells is associated with disease relapse. To distinguish between these possibilities in a tissue culture model, we used human ErbB2-positive breast cancer cells BT474 and their trastuzumab-resistant variant BT474TR that we generated by exposing BT474 to trastuzumab in culture for an extended time period and expanding the surviving cells [8]. We found that when grown in the absence of adhesion of the ECM (conditions that mimic 3D breast tumor growth [8, 14]), BT474 and BT474TR cells produce similar Irf6 levels (Fig. 3a). Thus, selection for trastuzumab resistance does not seem to be associated with increased Irf6 expression in ErbB2-positive breast cancer cells. We further generated a variant of BT474 cells BT474Irf6 stably overproducing exogenous hemagglutinin (HA)-tagged Irf6 by infecting BT474 cells with HA-Irf6-encoding retrovirus (Fig. 3b). We noticed that while trastuzumab treatment resulted in a significant loss of “BT474control” cells (a variant of BT474 cells infected with a control retrovirus) cultured without adhesion to the ECM, trastuzumab treatment did not trigger any significant changes in the number of “BT474Irf6” cells cultured under these conditions (Fig. 3c). Thus, stable Irf6 overexpression in ErbB2-positive breast cancer cells that do not die despite the presence of increased Irf6 levels renders the cells trastuzumab resistant. These data are consistent with our findings that relatively high Irf6 levels in the patients’ tumors are associated with disease relapse, i.e., trastuzumab resistance of these tumors.
Increased Irf6 expression in cultured ErbB2-positive breast cancer cells is associated with trastuzumab resistance a, b Indicated cell lines were cultured detached from the ECM for 24 h and assayed for Irf6 levels by western blotting. The membrane in (b) was also probed with the anti-HA antibody to detect HA-tagged Irf6. GAPDH served as a loading control. c Indicated cell lines were placed in suspension culture in the absence or in the presence of 5 μg/ml trastuzumab (TZ) for the indicated periods of time and counted. TZ-dependent change in cell number was calculated as the difference between the number of treated and that of the untreated cells. Negative numbers signify cell loss. The numbers represent the average of 9 (in the case of the 48-h treatment), 8 (in the case of the 72-h treatment), 4 (in the case of the 96-h treatment), and 5 (in the case of the 120-h treatment) independent experiments plus SE. *p˂0.05
High EGFR levels in the tumors before and after neoadjuvant therapies are associated with disease relapse
Since ErbB2-mediated EGFR upregulation protects breast cancer cells from anoikis at least in part, by downregulating the pro-apoptotic protein Perp [9], we decided to perform IHC analysis of EGFR levels in the breast tumor samples collected before and after neoadjuvant treatments. We first demonstrated that similar to what was published by others [33], our IHC conditions allowed us to detect positive EGFR staining in benign breast ductal epithelium (membranous), colonic epithelium (membranous), renal tubules (cytoplasmic), liver ducts (cytoplasmic), lung pneumocytes (membranous), skin (membranous), tonsil epithelium (membranous), and thyroid gland (membranous) (Supplementary Fig. 2). Similar to others [33], we did not observe EGFR in the tonsil lymphocytes or other stromal cells within the respective tissues (Supplementary Fig. 2). As expected [35], in the case of the breast tumor samples that we used EGFR displayed predominantly membrane localization in the tumor cells in all cases (Fig. 4b–d). We found that the percentage of EGFR-positive cells in the tumor samples is increased approximately sevenfold following trastuzumab-based treatments (on average, from 6 to 44%) in 25% of cases (Fig. 4a, b). The increase in EGFR levels was unequivocal in each case, but the overall effect was not statistically significant since the degree of increase strongly varied between patients, e.g., in some cases that the increase was from 0 to 10% and in others, from 2 to 95%. We further observed that the percentage of EGFR-positive cells in question is decreased approximately eightfold (on average, from 39 to 5%) in 21% of tumor samples following trastuzumab-based treatments. The latter change was statistically significant (Fig. 4a, c). The percentage of EGFR-positive cells was similar before and after the treatments in 54% of the samples (Fig. 4a, d). The decrease in the percentage of EGFR-positive cancer cells following the treatments did not correlate with relapse-free survival (not shown). In contrast, the increase in the percentage of EGFR-positive cells in the tumors following the treatments was significantly associated with a decreased relapse-free survival: p value = 0.013, HR = 5.6 (95% CI 1.4–21.6) (Fig. 4e). 46% of patient samples obtained before neoadjuvant therapies and 48% of patients’ samples collected after the therapies displayed EGFR-positive cells (Fig. 5a). Remarkably, patients whose tumor samples obtained before the therapies showed any percentage of EGFR-positive cancer cells had a significantly higher chance of disease relapse than the patients with the EGFR-negative tumors [p value = 0.027, HR = 3.7 (95% CI 1.1–13.0)] (Fig. 5a, b). In addition, the presence of any levels of EGFR-positive cells in the post-treated samples was significantly associated with reduced relapse-free survival [p-value = 0.037, HR = 3.5 (95% CI 1.02–12.3)] (Fig. 5a, c). Thus, the presence of EGFR in the breast tumor cells before or after neoadjuvant therapies or an increase in the percentage of EGFR-positive cells in the tumors following these treatments is associated with a higher chance of disease relapse.
Increased EGFR levels in patients’ tumors following neoadjuvant therapies are associated with disease relapse. a Percentage of EGFR-positive cells in patients’ tumor samples collected before and after the therapies. b–d Representative samples obtained from patients before (left) and after (right) the treatments are shown. The samples were stained with the anti-EGFR antibody (brown) and counterstained with hematoxylin (blue). Examples of an increase (b), decrease (c), or lack of change (d) in the EGFR levels are shown. e Kaplan–Meier analysis-based estimation of probabilities of patients’ relapse-free survival depending on whether or not % of EGFR-positive cells in the tumor samples is increased following the therapies. One patient that received neoadjuvant FEC-D alone was not included in the analysis
EGFR presence in patients’ tumors before or after neoadjuvant therapies is associated with disease relapse. a Percentage of patients with EGFR-positive cells in the tumor samples collected before and after the therapies. b, c Kaplan–Meier analysis-based estimation of probabilities of patients’ relapse-free survival depending on whether the tumor samples collected before (b) or after (c) the therapies display any EGFR levels. d The results of the multivariate analysis of the changes of HR of relapse in the case of continuous increase (from 0 to 12%) of % of Irf6-positive cells and that (from 0 to 95%) of % of EGFR-positive cells in the samples collected after the therapies
Since Irf6 and EGFR levels in the tumor cells following neoadjuvant therapies significantly correlated with disease relapse in the univariate analyses, we have conducted a multivariate analysis using Irf6 and EGFR levels in the post-treated tumors as continuous variables. We found that in the case of Irf6, the HR of relapse increases by 19.8% per increase of the percentage of Irf6-positive cells in the indicated samples by each percent (from 0 to 12) with the initial HR being 1.198 (95% CI 1.015, 1.424), p value = 0.0082 (Fig. 5d). In the case of EGFR, the HR of relapse increased by 2.4% per increase of the percentage of EGFR-positive cells in the indicated samples by each percent (from 0 to 95) with the initial HR being 1.024 (95% CI 1.006, 1.043), p value = 0.0323 (Fig. 5d). Thus, increased levels of both Irf6 and EGFR in the tumors following neoadjuvant trastuzumab-based therapies significantly correlated with reduced disease-free survival in the multivariate analysis.
High Irf6 and EGFR levels in the tumors are associated with reduced patient survival
We further established as outlined above that the presence of relatively high Irf6 levels in the tumors after neoadjuvant therapies was significantly associated with reduced patients’ overall survival: p-value = 0.013, hazard ratio (HR) = 5.8, (95% CI 1.4–23.4.1) (Fig. 6a). We also noticed that the increase in the percentage of EGFR-positive cells in the tumors following the therapies was significantly associated with a decreased patients’ overall survival: p value = 0.003, HR = 13.2 (95% CI 2.3–74.6) (Fig. 6b). In addition, patients whose tumor samples obtained before or after the therapies showed any percentage of EGFR-positive cancer cells had a significantly lower chance of survival than the patients with the EGFR-negative tumors (p value = 0.04, HR = 5.4 (95% CI 1.1–21.1) in the case of the patients with EGFR-positive tumors before the therapies; p value = 0.017, HR = 13.1 (95% CI 1.6–107.2) in the case of the patients with EGFR-positive tumors after the therapies) (Fig. 6c, d). Thus, high Irf6 and EGFR levels in the tumors tend to be associated with reduced overall survival of the indicated patients.
Increased Irf6 and EGFR levels in patients’ tumors following neoadjuvant therapies are associated with reduced patients’ overall survival. a Kaplan–Meier analysis-based estimation of probabilities of patients’ overall survival depending on whether % of Irf6-positve cells in the tumor samples collected after the therapies are higher or lower than 6%. b Kaplan–Meier analysis-based estimation of probabilities of patients’ overall survival depending on whether or not % of EGFR-positive cells in the tumor samples is increased following the therapies. One patient that received neoadjuvant FEC-D alone was not included in the analysis. c, d Kaplan–Meier analysis-based estimation of probabilities of patients’ overall survival depending on whether the tumor samples collected before (c) or after (d) the therapies display any EGFR levels
Perp levels in the tumors before and after neoadjuvant therapies do not correlate with disease relapse
Since ErbB2-driven downregulation of the pro-apoptotic protein Perp inhibits breast cancer cell anoikis [9], we decided to perform IHC analysis of Perp levels in the breast tumor samples collected before and after trastuzumab-based neoadjuvant treatments. We first validated the Perp IHC by demonstrating cytoplasmic Perp staining in the cardiac myocytes, as described by the manufacturer (see “Patients and Methods” section) and nuclear and cytoplasmic Perp staining in the skin keratinocytes as published by others [33] (Supplementary Fig. 3). In the case of the breast cancer samples collected by us, Perp displayed mainly nuclear localization in the tumor cells (Fig. 7b–d). The percentage of Perp-positive cells was increased 1.9-fold further to neoadjuvant treatments (on average, from 36 to 67%) in 29% cases (Fig. 7a, b). This increase was not statistically significant (Fig. 7a). We also observed that the percentage of Perp-positive cells is decreased 2.6-fold (on average, from 87 to 34%) in 42% of cases following these therapies (Fig. 7a, c) in a statistically significant manner. The percentage of Perp-positive cells was similar before and after the treatments in 29% of the tumors (Fig. 7a, d). Neither the increase nor the decrease in the percentage of Perp-positive tumor cells following the treatments was associated with a statistically significant correlation with patients’ relapse-free survival (not shown). Likewise, the presence of Perp in the tumor cells before or after the treatment did not correlate with disease relapse (not shown).
Neoadjuvant therapy-dependent changes in Perp levels in patients’ tumors before and after the therapies. a Percentage of Perp-positive cells in patients’ tumor samples collected before and after the therapies. b–d Representative samples obtained from patients before (left) and after (right) the treatments are shown. The samples were stained with the anti-Perp antibody (brown) and counterstained with hematoxylin (blue). Examples of an increase (b), decrease (c), or lack of change (d) in the Perp levels are shown
The presence of lymph node metastases at tumor resection is associated with disease relapse
We observed that patients’ clinicopathological characteristics, such as age at diagnosis, therapy regimen, clinical tumor stage, and grade did not correlate significantly with disease relapse (not shown). We also noticed that the presence of lymph node metastases at tumor resection is significantly associated with breast cancer relapse (Supplementary Fig. 4a). The log-rank test showed a statistically significant (p = 0.006) difference between the relapse-free survival curves for the lymph node status. 60.87% of patients with the tumor-positive lymph nodes relapsed while none of the patients with the tumor-free lymph nodes displayed disease relapse. The latter observation well agrees with what was published by others [36]. Due to the relatively small size of our patient cohort and since none of the patients in the cohort with the lymph node-negative disease experienced disease relapse, we could not include tumor lymph nodes in the multivariate model described above. Nevertheless, we noticed that the patient group with the tumor-positive lymph nodes that relapsed contained a higher percentage of patients with increased Irf6 or EGFR levels than respective patient groups with the tumor-positive lymph nodes that did not relapse. The indicated differences were statistically significant in the case of both Irf6 and EGFR (Supplementary Fig. 4b, c). Hence, relatively high Irf6 and EGFR levels tend to be associated with disease relapse in the case of the patients with the tumor-positive lymph nodes.
In summary, we have established that relatively high Irf6 levels in ErbB2-positive breast cancer cells following neoadjuvant therapies that precede further adjuvant trastuzumab treatment significantly correlates with increased risk of breast cancer relapse and reduced patient survival. Moreover, we have observed that the presence of any EGFR levels in the indicated malignant cells before or after the treatments or an increase in these levels following the therapies in question is significantly associated with disease relapse and reduced patient survival.
Discussion
We have demonstrated that an increase in the Irf6 levels in ErbB2-positive breast tumors following neoadjuvant trastuzumab-based breast cancer therapies is associated with disease relapse and reduced patients’ survival. To our knowledge, Irf6 has never been studied in this context.
We observed previously that ErbB2 blocks cancer cell anoikis by downregulating Irf6 in the tumor cells, and that enforced Irf6 upregulation kills these cells [8]. Furthermore, we have shown here that increased Irf6 expression in cultured ErbB2-positive breast cancer cells is associated with trastuzumab resistance. Thus, the signals that render the cells resistant to the presence of increased Irf6 levels also make them resistant to trastuzumab in culture. These data are consistent with our observation that the presence of relatively high Irf6 levels in cancer cells is associated with disease relapse and reduced patients’ survival. Hence, the mechanisms allowing the cells to survive in the presence of high Irf6 levels could represent novel targets for therapies aimed at overcoming breast cancer cell trastuzumab resistance.
We also observed that the presence of EGFR in breast tumor cells before or after neoadjuvant treatments or EGFR upregulation in such cells following the treatments is associated with disease relapse and reduced patients’ survival. The fact that EGFR presence in breast cancer cells correlates with disease relapse is consistent with a well-established oncogenic role of this protein [35]. Moreover, EGFR upregulation in ErbB2-positive breast cancer cells was shown to be associated with their trastuzumab resistance in mice [37]. Finally, it was demonstrated that EGFR overexpression in the primary ErbB2-positive breast tumors is associated with reduced rate of patients’ disease-free survival [38]. Importantly, to our knowledge, the connection between EGFR tumor levels before and after neoadjuvant trastuzumab-based therapies and disease outcome has never been made in the context of neoadjuvant therapies of stage II and III ErbB2-positive breast cancers.
Another important therapeutic implication of our findings is based in the fact that EGFR is a relatively easily targetable receptor, and several EGFR inhibitors are presently used in the clinic for cancer treatment or are being investigated in pre-clinical and clinical studies [39]). Hence, testing whether treatment of patients with both ErbB2- and EGFR-positive breast tumors with one of these EGFR inhibitors improves the effects of trastuzumab on disease outcomes could represent an important direction for the studies aimed at enhancing the efficacy of trastuzumab-based breast cancer therapies.
To test whether our results regarding Irf6 and EGFR protein levels in breast tumors as potential predictors of breast cancer trastuzumab response are consistent with those on the levels of respective mRNAs, we utilized an online tool for identification of mRNA expression-based predictive biomarkers using gene expression microarray analysis of various breast tumors sets [40]. One of these sets contains surgical early-stage breast tumor samples derived from patients prior to adjuvant trastuzumab treatment [41]. In agreement with our observations, we established using the indicated online tool that increased Irf6 and EGFR mRNA levels in the samples derived from trastuzumab-treated patients are associated with disease relapse within 5 years in a statistically significant manner (Supplementary Fig. 5).
It has to be noted that in addition to the effects of ErbB2 on Irf6, Perp, and EGFR, ErbB2-driven breast cancer anoikis resistance is likely regulated by other mechanisms, e.g., ErbB2-induced downregulation of a pro-apoptotic protein Bim [42] or ErbB2-dependent upregulation of integrin alpha 5 [43]. Testing whether elements of these mechanisms can serve as potential predictors of breast cancer trastuzumab response represent an important direction for our future studies.
In summary, our previous analysis of ErbB2-dependent mechanisms of breast cancer cell anoikis resistance identified three mediators of this resistance, Irf6, EGFR, and Perp [8, 9]. We have now demonstrated for the first time that the levels of two of these proteins, Irf6 and EGFR, in breast tumors of patients that receive trastuzumab-based neoadjuvant therapies correlate with disease relapse and reduced patients’ survival. Thus, further studies aimed at establishing whether high Irf6 and/or EGFR tumor levels predict the relapse of ErbB2-postitve breast cancers in patients receiving neoadjuvant trastuzumab-based treatments are feasible. Identification of such patients could allow oncologists to improve disease management.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bethune GC, van Zanten DV, MacIntosh RF, Rayson D, Younis T, Thompson K, Barnes PJ (2015) Impact of the 2013 American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 (HER2) testing of invasive breast carcinoma: a focus on tumours assessed as ‘equivocal’ for HER2 gene amplification by fluorescence in-situ hybridization. Histopathology 67:880–887. https://doi.org/10.1111/his.12723
Bartsch R, Wenzel C, Steger GG (2007) Trastuzumab in the management of early and advanced stage breast cancer. Biologics 1:19–31
Untch M, Fasching PA, Konecny GE, Hasmuller S, Lebeau A, Kreienberg R, Camara O, Muller V, du Bois A, Kuhn T et al (2011) Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol 29:3351–3357. https://doi.org/10.1200/JCO.2010.31.4930
Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, Bauerfeind I, Hilfrich J, Eidtmann H, Gerber B et al (2010) Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol 28:2024–2031. https://doi.org/10.1200/JCO.2009.23.8451
Triulzi T, Bianchi GV, Tagliabue E (2016) Predictive biomarkers in the treatment of HER2-positive breast cancer: an ongoing challenge. Future Oncol 12:1413–1428. https://doi.org/10.2217/fon-2015-0025
Telli ML, Hunt SA, Carlson RW, Guardino AE (2007) Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol 25:3525–3533. https://doi.org/10.1200/Jco.2007.11.0106
Drucker A, Skedgel C, Virik K, Rayson D, Sellon M, Younis T (2008) The cost burden of trastuzumab and bevacizumab therapy for solid tumours in Canada. Curr Oncol 15:136–142. https://doi.org/10.3747/co.v15i3.249
Khan IA, Yoo BH, McPhee M, Masson O, Surette A, Dakin-Hache K, Younis T, Bethune G, Rosen KV (2018) ErbB2-driven downregulation of the transcription factor Irf6 in breast epithelial cells is required for their 3D growth. Breast Cancer Res 20:151
Khan IA, Yoo BH, Masson O, Baron S, Corkery D, Dellaire G, Attardi LD, Rosen KV (2016) ErbB2-dependent downregulation of a pro-apoptotic protein Perp is required for oncogenic transformation of breast epithelial cells. Oncogene 35:5759–5769. https://doi.org/10.1038/onc.2016.109
Jacks T, Weinberg RA (2002) Taking the study of cancer cell survival to a new dimension. Cell 111:923–925. https://doi.org/10.1016/s0092-8674(02)01229-1
Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS (2002) The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini (vol 111, pg 29, 2002). Cell 111:757–757. https://doi.org/10.1016/S0092-8674(02)01163-7
Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW (2002) Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci 115:39–50
Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ (2002) beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell 2:205–216. https://doi.org/10.1016/s1535-6108(02)00125-3
Debnath J, Brugge JS (2005) Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer 5:675–688. https://doi.org/10.1038/nrc1695
Frisch SM, Francis H (1994) Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 124:619–626. https://doi.org/10.1083/jcb.124.4.619
Freedman VH, Shin SI (1974) Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell 3:355–359. https://doi.org/10.1016/0092-8674(74)90050-6
Rosen K, Rak J, Jin J, Kerbel RS, Newman MJ, Filmus J (1998) Downregulation of the pro-apoptotic protein Bak is required for the ras-induced transformation of intestinal epithelial cells. Curr Biol 8:1331–1334. https://doi.org/10.1016/S0960-9822(07)00564-7
Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE (2004) EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene 23:1448–1456
Rosen K, Rak J, Leung T, Dean NM, Kerbel RS, Filmus J (2000) Activated Ras prevents downregulation of Bcl-X(L) triggered by detachment from the extracellular matrix. A mechanism of Ras-induced resistance to anoikis in intestinal epithelial cells. J Cell Biol 149:447–456. https://doi.org/10.1083/jcb.149.2.447
Scotlandi K, Maini C, Manara MC, Benini S, Serra M, Cerisano V, Strammiello R, Baldini N, Lollini PL, Nanni P et al (2002) Effectiveness of insulin-like growth factor I receptor antisense strategy against Ewing’s sarcoma cells. Cancer Gene Ther 9:296–307
Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE (2004) CEACAM6 gene silencing impairs anoikis resistance and in vivo metastatic ability of pancreatic adenocarcinoma cells. Oncogene 23:465–473
Berezovskaya O, Schimmer AD, Glinskii AB, Pinilla C, Hoffman RM, Reed JC, Glinsky GV (2005) Increased expression of apoptosis inhibitor protein XIAP contributes to anoikis resistance of circulating human prostate cancer metastasis precursor cells. Cancer Res 65:2378–2386
Attardi LD, Reczek EE, Cosmas C, Demicco EG, McCurrach ME, Lowe SW, Jacks T (2000) PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev 14:704–718
Ihrie RA, Attardi LD (2005) A new Perp in the lineup: linking p63 and desmosomal adhesion. Cell Cycle 4:873–876. https://doi.org/10.4161/cc.4.7.1836
Ihrie RA, Reczek E, Horner JS, Khachatrian L, Sage J, Jacks T, Attardi LD (2003) Perp is a mediator of p53-dependent apoptosis in diverse cell types. Curr Biol 13:1985–1990. https://doi.org/10.1016/j.cub.2003.10.055
Beaudry VG, Jiang D, Dusek RL, Park EJ, Knezevich S, Ridd K, Vogel H, Bastian BC, Attardi LD (2010) Loss of the p53/p63 regulated desmosomal protein Perp promotes tumorigenesis. PLoS Genet 6:e1001168. https://doi.org/10.1371/journal.pgen.1001168
Taniguchi T, Ogasawara K, Takaoka A, Tanaka N (2001) IRF family of transcription factors as regulators of host defense. Annu Rev Immunol 19:623–655. https://doi.org/10.1146/annurev.immunol.19.1.623
Lin Y, Xu D, Li X, Liu C, Liu X, Huang S, Huang Y, Liu X (2016) Upregulation of interferon regulatory factor 6 promotes neuronal apoptosis after traumatic brain injury in adult rats. Cell Mol Neurobiol 36:27–36. https://doi.org/10.1007/s10571-015-0217-3
Bailey CM, Margaryan NV, Abbott DE, Schutte BC, Yang B, Khalkhali-Ellis Z, Hendrix MJ (2009) Temporal and spatial expression patterns for the tumor suppressor Maspin and its binding partner interferon regulatory factor 6 during breast development. Dev Growth Differ 51:473–481. https://doi.org/10.1111/j.1440-169X.2009.01110.x
Wiesen J, Werb Z (2000) Proteinases, cell cycle regulation, and apoptosis during mammary gland involution (minireview). Mol Reprod Dev 56:534–540. https://doi.org/10.1002/1098-2795(200008)56:4%3c534::AID-MRD12%3e3.0.CO;2-O
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of NCIEWGoCD (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100:229–235. https://doi.org/10.1007/s10549-006-9242-8
Liu Z, Li H, Derouet M, Filmus J, LaCasse EC, Korneluk RG, Kerbel RS, Rosen KV (2005) ras Oncogene triggers up-regulation of cIAP2 and XIAP in intestinal epithelial cells: epidermal growth factor receptor-dependent and -independent mechanisms of ras-induced transformation. J Biol Chem 280:37383–37392
Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A et al (2015) Proteomics. Tissue-based map of the human proteome. Science 347:1260419. https://doi.org/10.1126/science.1260419
Botti E, Spallone G, Moretti F, Marinari B, Pinetti V, Galanti S, De Meo PD, De Nicola F, Ganci F, Castrignano T et al (2011) Developmental factor IRF6 exhibits tumor suppressor activity in squamous cell carcinomas. Proc Natl Acad Sci USA 108:13710–13715. https://doi.org/10.1073/pnas.1110931108
Yarden Y, Sliwkowski MX (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2:127–137. https://doi.org/10.1038/35052073
Shimizu C, Masuda N, Yoshimura K, Tsuda H, Mano M, Ando M, Tamura K, Fujiwara Y (2009) Long-term outcome and pattern of relapse after neoadjuvant chemotherapy in patients with human epidermal growth factor receptor 2-positive primary breast cancer. Jpn J Clin Oncol 39:484–490. https://doi.org/10.1093/jjco/hyp052
Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, Arteaga CL (2007) Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res 13:4909–4919. https://doi.org/10.1158/1078-0432.CCR-07-0701
Lee HJ, Seo AN, Kim EJ, Jang MH, Kim YJ, Kim JH, Kim SW, Ryu HS, Park IA, Im SA et al (2015) Prognostic and predictive values of EGFR overexpression and EGFR copy number alteration in HER2-positive breast cancer. Br J Cancer 112:103–111. https://doi.org/10.1038/bjc.2014.556
Ayati A, Moghimi S, Salarinejad S, Safavi M, Pouramiri B, Foroumadi A (2020) A review on progression of epidermal growth factor receptor (EGFR) inhibitors as an efficient approach in cancer targeted therapy. Bioorg Chem 99:103811. https://doi.org/10.1016/j.bioorg.2020.103811
Fekete JT, Gyorffy B (2019) ROCplot.org: validating predictive biomarkers of chemotherapy/hormonal therapy/anti-HER2 therapy using transcriptomic data of 3,104 breast cancer patients. Int J Cancer 145:3140–3151. https://doi.org/10.1002/ijc.32369
Sircoulomb F, Bekhouche I, Finetti P, Adelaide J, Ben Hamida A, Bonansea J, Raynaud S, Innocenti C, Charafe-Jauffret E, Tarpin C et al (2010) Genome profiling of ERBB2-amplified breast cancers. BMC Cancer 10:539. https://doi.org/10.1186/1471-2407-10-539
Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, Muthuswamy SK, Brugge JS (2003) Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol 5:733–740. https://doi.org/10.1038/ncb1026
Haenssen KK, Caldwell SA, Shahriari KS, Jackson SR, Whelan KA, Klein-Szanto AJ, Reginato MJ (2010) ErbB2 requires integrin alpha5 for anoikis resistance via Src regulation of receptor activity in human mammary epithelial cells. J Cell Sci 123:1373–1382. https://doi.org/10.1242/jcs.050906
Funding
These studies were supported by the Canadian Institutes of Health Research under the Project Grant 156015 (PI-KVR), and by Beatrice Hunter Cancer Research Institute under the Seed Grant (KVR and GB-co-PIs).
Author information
Authors and Affiliations
Contributions
KVR, GB, and TY designed the study, TR, JS, and GB acquired patient samples and clinical data, AS and GB generated IHC data, BHY performed the tissue culture studies, KM performed statistical analysis of the data, AS, TY, GB, and KVR analyzed results of the study, and KVR and GB supervised the study. KVR wrote this manuscript as a result of numerous discussions with the authors. All authors read the manuscript and approved its content.
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethical approval
Studies involving human tumor samples were approved by Nova Scotia Health Authority Research Ethics Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Surette, A., Yoo, B.H., Younis, T. et al. Tumor levels of the mediators of ErbB2-driven anoikis resistance correlate with breast cancer relapse in patients receiving trastuzumab-based therapies. Breast Cancer Res Treat 187, 743–758 (2021). https://doi.org/10.1007/s10549-021-06164-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06164-0