Abstract
Purpose
This study compares the sensitivity of dedicated breast positron emission tomography (DbPET) and whole body positron emission tomography (WBPET) in detecting invasive breast cancer based on tumor size and biology. Further, we explored the relationship between maximum standardized uptake value (SUVmax) of DbPET and biological features of the tumor.
Methods
A total of 639 invasive breast cancer lesions subjected to both DbPET and WBPET before surgery, between January 2016 and May 2019, were included in the study. The sensitivity of DbPET and WBPET in detection and the biology of the tumor according to the clinicopathological features were retrospectively evaluated.
Results
The overall sensitivity of DbPET was higher than that of WBPET (91.4% vs. 80.3%, p < 0.001). Subcentimetric tumors were significant (80.9% vs. 54.3%, p < 0.001). Regardless of the nuclear grade, DbPET could detect more lesions than WBPET. The SUVmax was positively correlated with tumor size (R = 0.395, p < 0.001) and the nuclear grade (p < 0.001). Luminal A-like breast cancer had significantly lower SUVmax values than the other subtypes (p < 0.001).
Conclusions
DbPET is superior to WBPET in the detection of subcentimetric, low-grade breast cancers. Further, by using SUVmax, DbPET can distinguish luminal A-like breast cancer from the other subtypes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Screening mammography increased the detection of breast cancers < 2 cm in size, thereby reducing their associated mortality [1, 2]. Disease-free survival of patients with stage T1a/T1b breast cancer is more favorable than those with T1c disease, and the 10-year risk of breast cancer death is < 5% for women with a subcentimetric disease [1, 3]. Therefore, it is important to detect breast cancer at an early stage (< 1 cm in size).
Recently, the use of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) has increased in breast cancer diagnostics. FDG PET, which reflects the glucose metabolism of malignancies, visualizes the primary breast tumor, lymph node, and distant metastases and is used for cancer staging [4]. In addition to the detection of malignant tumors, the maximum standardized uptake value (SUVmax) on whole body PET (WBPET) predicts the grade and prognosis of patients with breast cancer [5,6,7,8]. However, WBPET has the disadvantage of false-negative results for small (< 1 cm) and low-grade breast cancers [9].
Dedicated breast PET (DbPET) is a molecular breast imaging system with a high spatial resolution that is believed to detect small breast cancers. Detection of subcentimetric breast tumors might lead to the identification of multiple occult lesions that evade conventional diagnosis. Breast cancer presents as multiple ipsilateral and bilateral lesions. Previous studies have reported a frequency of 5.2% to 6.4% for multiple ipsilateral breast cancers [10,11,12,13] and 1.4% to 5.4% for bilateral breast cancers [14,15,16]. Accurate diagnosis of multiple breast cancers is essential for deciding the extent of surgical procedures for adequate clearance of the tumor.
Previous literature on the sensitivity of DbPET is limited to small clinical studies [17,18,19,20]. In this study, we aimed to compare the sensitivity of DbPET and WBPET in detecting invasive breast cancer based on tumor size and biology in a large cohort.
Materials and methods
Patients
The study enrolled 593 consecutive patients with primary breast cancer who were subjected to both DbPET and WBPET before treatment at the Hiroshima University Hospital between January 2016 and May 2019. Hiroshima University conducts DbPET on all breast cancer cases, except in instances where the patient refuses. Female patients aged > 20 years with invasive breast cancer that was histologically confirmed using a specimen of primary surgery or needle-biopsy before neoadjuvant chemotherapy were included in the study. Patients with non-invasive breast cancer or local recurrence were excluded. The Institutional Review Board approved this study. All procedures performed involving human participants were in accordance with the ethical standards of the institutional research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this retrospective study, the need for formal consent was waived.
WBPET and DbPET examinations
Patients fasted for at least 4 h before the administration of FDG. WBPET scans were performed using a Discovery ST16 integrated PET/computed tomography (CT) scanner (GE Healthcare, Little Chalfont, UK) 1 h after the injection of 3–3.7 MBq/kg body weight of FDG. For attenuation correction and localization of lesions identified by PET, low-dose non-enhanced CT images with sections 3 to 4 mm thick were obtained from the head to the pelvic floor of each patient according to a standard CT imaging protocol. Immediately after CT imaging, the identical axial field of view (FOV) (154 mm) was scanned using PET for 2 to 3 min per table position depending on the patient's condition and the scanner performance. WBPET/CT studies were performed with patients in the supine position and normal tidal breathing. Acquired data were reconstructed as 128 × 128 matrix images (pixel size, 4.7 × 3.25 mm) using Fourier rebinning and ordered subset expectation–maximization algorithms.
The subsequent DbPET imaging studies started approximately 1.5 h after FDG injection. DbPET imaging was performed with the patient in the prone position using an Elmammo scanner (Shimadzu, Kyoto, Japan). The transaxial effective FOV was 185 × 156.5 mm, the scan time was 7 min for each breast, and the acquired data were reconstructed as 236 × 236 matrix images (pixel size, 0.78 × 0.78 mm) using a three-dimensional dynamic row-action maximum likelihood algorithm.
PET image evaluations and quantifications of SUVmax were performed using a Xeleris workstation (Version 1.1452, GE Healthcare). For the visual assessment, increased uptake of FDG with an intensity higher than that of the surrounding tissues and that is not explainable by physiological processes was considered positive for tumors. Thereafter, the region of interest (ROI) was placed over the tumor, and the ROI with multiple cross-sectional images was used for standardized uptake value measurements. Attenuation correction of DbPET was carried out using the homogeneous soft tissue composed of mammary and adipose tissue. Lesions completely or partially outside the FOV on DbPET were excluded from the analysis of SUVmax. If the breast cancer lesions were unclear on both DbPET and WBPET, they were assessed visually and removed from the SUV analysis. In DbPET, the SUVmax was measured in both the primary lesion and the background. Background uptake was measured in the ipsilateral normal breast tissue. Visual assessment, interpretation of the images, and data analysis were performed by a nuclear medicine physician who was aware of the patients’ clinical history provided by the referring physician, but blinded to the results of other imaging studies. For confirmation, all PET images were consensus-read by a breast cancer specialist.
Histological examination
Histological evaluation of the surgical specimens was performed and reported according to the Union for International Cancer Control (UICC) TNM Classification of Malignant Tumors [21]. For patients who underwent neoadjuvant chemotherapy, the pre-treatment biopsy specimens were evaluated. Tumor size was assessed based on the pathological size for tumors without neoadjuvant chemotherapy and clinical size (size on the ultrasonography or magnetic resonance imaging) for neoadjuvant chemotherapy cases. Estrogen receptor (ER) and human epidermal growth factor receptor type 2 (HER2) status were assessed according to the guidelines of the American Society of Clinical Oncology/College of American Pathologists [22, 23]. The molecular subtypes of breast cancer were classified as luminal (ER+/HER2−), ER+/HER2+, ER−/HER2+, or triple-negative (ER−/HER2−). The luminal breast cancer types were classified as luminal A-like (Ki-67 labeling index < 20%) and luminal B-like (Ki-67 labeling index ≥ 20%) on the basis of St. Gallen International Expert Consensus [24].
Statistical analysis
The clinicopathological characteristics of the patients were represented as medians (interquartile range) for continuous variables and as numbers (%) for categorical variables. The differences in the sensitivity for tumor detection between DbPET and WBPET were analyzed using McNemar’s Test. The comparison of SUVmax among nuclear grades or subtypes was analyzed using Tukey's test. A 1:1 paired matching according to propensity scores, including age, tumor size, node metastasis, and nuclear grade, was applied separately on luminal A-like and non-luminal A-like groups. The SUVmax values in DbPET were classified into two groups based on the median value (6.8). Frequencies were compared using χ2 test, whereas continuous variables were compared using the Mann–Whitney U test.
All statistical analyses were performed using JMP® version 14.0 (SAS Institute, Cary, NC, USA), and a p value < 0.05 was considered significant in all comparisons.
Results
Patient characteristics
The median age was 56 years (range: 22–90 years). Overall, 593 patients had 639 invasive breast cancers. Multicentric and multifocal tumors were evaluated as separate tumors. Among the 639 invasive breast cancers, 31.1% were T1mi/T1a/T1b (≤ 1 cm) tumors (Table 1). Histologically, nuclear grade 1 was seen in 23.0%, grade 2 in 37.4%, and grade 3 in 39.4% of the lesions. The molecular subtype distribution in patients was as follows: luminal A-like in 210 (32.9%), luminal B-like in 282 (44.1%), ER+/HER2+ in 57 (8.9%), ER−/HER2+ in 18 (2.8%), and triple-negative in 56 (8.8%). A total of 89 patients (with 93 invasive breast cancers) received neoadjuvant chemotherapy.
Sensitivity of breast cancer detection by DbPET and WBPET
The sensitivity of DbPET was higher than that of WBPET (91.4% vs. 80.3%, p < 0.001). The difference in sensitivity according to tumor size was significant in subcentimetric tumors (80.9% vs. 54.3%, p < 0.001): T1mi (89.2% vs. 62.2%, p = 0.004), T1a (76.9% vs. 38.5%, p < 0.001), and T1b (80.0% vs. 59.1%, p < 0.001) (Table 2). The sensitivity of DbPET was higher than that of WBPET for all nuclear grades, and the difference was significant in lower grade tumors (Table 3). The sensitivity of DbPET was also significantly higher than that of WBPET in all the molecular subtypes except the ER+/HER2+ and ER−/HER2+ types (Table 3). In particular, the diagnostic sensitivity of DbPET was > 15% over that of WBPET in the luminal A-like subtype. Of the 639 lesions, 42 (6.6%) could not be detected by either DbPET or WBPET and 13 (2.0%) could be detected by WBPET, but not DbPET. Of the 42 lesions that could not be detected by either DbPET or WBPET, 30 lesions (71.4%) were ≤ 1 cm. Of the 13 lesions that could be detected by WBPET, but not DbPET, 10 (76.9%) were determined to be out of the FOV, and the remaining 3 lesions were less than 15 mm from the chest wall although they were in the FOV. Therefore, they were difficult to distinguish from noise.
Sensitivity of detection of additional ipsilateral and contralateral breast cancer
Of the 639 lesions examined in this study, 46 were found in addition to the primary lesion detected during screening or presentation of symptoms. Of those 46 lesions, 29 were ipsilateral, whereas 17 were contralateral. The sensitivity of DbPET and WBPET in detecting additional lesions was 62.1% and 37.9% for ipsilateral lesions and 70.6% and 58.8% for contralateral lesions, respectively. Four of the ipsilateral and two of the contralateral additional tumors were detected only by DbPET. Figure 1 shows a case with multiple ipsilateral breast cancers, and Fig. 2 shows a case with bilateral breast cancers.
A 47-year-old woman with two invasive ductal carcinomas in the left breast. a Maximum intensity projection image of whole body positron emission tomography (WBPET); b axial images of WBPET/computed tomography (CT) fusion; and c sagittal image of dedicated breast positron emission tomography (DbPET) showing massive 18F-fluorodeoxyglucose (FDG) uptake in the left breast (arrow). Only DbPET shows another small lesion in the left breast (arrowhead). *Axillary lymph node with abnormal uptake.
An 87-year-old woman with invasive ductal carcinoma in both breasts. a Maximum intensity projection image of whole body positron emission tomography (WBPET); b axial images of WBPET/computed tomography (CT) fusion; and c sagittal image of dedicated breast positron emission tomography (DbPET), showing massive 18F-fluorodeoxyglucose (FDG) uptake in the left breast (arrow). Only DbPET shows small lesion in the right breast (arrowhead)
Relationship between the SUVmax of DbPET and biological features of the tumor
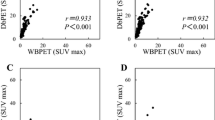
The SUVmax significantly correlated with the tumor size (p < 0.001) and the nuclear grade (Fig. 3a, b). The median SUVmax values of the luminal A-like, luminal B-like, ER+/HER2+, ER−/HER2+, and triple-negative subtypes were 4.8, 8.3, 9.8, 14.9, and 12.4, respectively (Fig. 3c, all values of p < 0.001 relative to luminal A-like). A comparison between lesion-to-background ratio (LBR) and SUVmax showed similar results (Fig. 4). The propensity score matching analysis using tumor size, node metastasis, and nuclear grade is summarized in Table 4. The tumor biological factors were balanced between luminal A-like and non-luminal A-like groups. Between the two groups with no difference in age, tumor size, node metastasis, and nuclear grade, the luminal A-like group had a significantly higher proportion of lower SUVmax of DbPET than the non-luminal A-like group (p = 0.045). DbPET distinguished the luminal A-like tumor subtype from other subtypes.
a, b Relationship between maximum standardized uptake value (SUVmax) and biological features of the tumor showing significant correlation with tumor size and nuclear grade. There were significant differences between nuclear grade 1 and 3 or 2 and 3. c SUVmax on dedicated breast positron emission tomography (DbPET) according to molecular subtypes showing significantly lower SUVmax for luminal A-like tumor. There were significant differences between luminal A-like tumor and each of the other subtypes
a, b Relationship between lesion-to-background ratio (LBR) and the biological features of the tumor showing a significant correlation with tumor size and nuclear grade. There were significant differences between nuclear grade 1 and 3 or 2 and 3. c LBR on dedicated breast positron emission tomography (DbPET) according to molecular subtypes showing significantly lower LBR for luminal A-like tumor. There were significant differences between luminal A-like tumor and each of the other subtypes
DbPET was superior to WBPET in detecting small and low-grade tumors. Therefore, we compared SUVmax with subtypes (luminal A-like vs non-luminal A-like) in a subpopulation of small (< 2 cm) and low-grade (nuclear grade 1 or 2) tumors (n = 270). The median SUVmax of the luminal A-like group (n = 149) was 5.0, and the median SUVmax of the non-luminal A-like group (n = 121) was 6.2. A significant difference (p = 0.007) in the SUVmax between the two groups in the subpopulation of small and low-grade tumors was observed.
Discussion
In this study, DbPET was shown to be more effective than WBPET in detecting small and low-grade breast cancers. Recently, availability and utilization of WBPET examinations are increasing in cancer management. WBPET has been reported to be useful in detecting distant metastases, staging, and determining the effect of treatment [25]. However, WBPET has low sensitivity for small and low-grade tumors because of its limited spatial resolution [9].
DbPET has a high-resolution design based on the proximity of the breast scanner and has been developed to address the problems of WBPET. DbPET is classified into opposite- and ring-type scanners [26]. The opposite-type DbPET, such as positron emission mammography (PEM), showed a higher sensitivity than WBPET for tumors < 1 cm (66.7% vs. 13.3%, p = 0.008) [18]. However, the difference in lesion-based sensitivity between the ring-type DbPET and WBPET was not found to be significant in a study of 179 breast cancers (92% vs. 88%, p = 0.06) [20]. This was probably because of the inclusion of only 28 (15.6%) tumors ≤ 1 cm in size. In the present study, a large cohort of 639 breast cancers was evaluated, and the overall sensitivity of DbPET was significantly higher than that of WBPET (91.4% vs. 80.3%, p < 0.001). In addition, the difference was significant in the 199 breast cancers of size ≤ 1 cm. A previous study reported that there were no significant differences in sensitivity between DbPET and WBPET for all nuclear grades [20]. In our study, DbPET could detect breast cancers better than WBPET for all nuclear grades. This large cohort study clearly establishes the superiority of DbPET over WBPET for detecting breast cancers, especially small and low-grade tumors.
WBPET cannot detect "small size" tumors, whereas DbPET cannot detect tumors that are "outside or at the edge of the FOV" [27]. Tumors close to the chest wall in the breasts of young, slim women tend to be out of the FOV in which lesions are difficult to detect with DbPET. This is a drawback of DbPET. We considered it appropriate to include these tumors for comparison of the performance of DbPET and WBPET; therefore, we did not exclude tumors outside the FOV. Furthermore, the edges of the FOV are often noisy, and there is a possibility that the noise will be measured as SUVmax for tumors that are close to the edge of the FOV. In this case, 92 tumors (14.4%) were close to the chest wall (within 15 mm from the edge of the FOV of DbPET) in magnetic resonance imaging (MRI) measurements. Attention should be paid to the reliability of SUVmax for these tumors. This study did not include non-cancer cases; therefore, it was not possible to evaluate false positives and false negatives in DbPET, but these measures have been previously reported [27, 28].
Diagnosis of small breast cancers may lead to overdiagnosis [1]. However, the prognosis of subcentimetric breast cancer is excellent [3, 29]. A recent study demonstrated that mammography screening reduced the 10-year breast cancer mortality by 41%, independent of the systemic treatment progress [2]. In addition, DbPET can identify small multiple lesions and contribute to surgical decision-making in a preoperative setting. Searching for multiple lesions allows appropriate resection margins during surgery to be set. In our findings, 46 of the 639 lesions were discovered as additional lesions, 6 of which were detected only by DbPET. These patients underwent complete resection for better clearance and are expected to have a lower risk of recurrence.
Ring-type DbPET has the advantage of measuring SUVmax compared with PEM. Several studies reported that the SUVmax of WBPET predicted the biology and prognosis of breast cancer [6,7,8]. DbPET, with a high spatial resolution, may predict the tumor biology more accurately. A previous study reported that the SUVmax of DbPET was correlated with the histologic grade and molecular subtype of breast cancers and was lowest for luminal A-like tumors (p < 0.001) [30]. In the propensity score-matched patients, the SUVmax values of the luminal A-like group were lower than those of non-luminal A-like group. The SUVmax values of luminal A-like tumors were lower than those of the other subtypes in a subpopulation of small and low-grade tumors. The SUVmax of DbPET may be a non-invasive indicator for determining an appropriate systemic treatment strategy for invasive breast cancer.
This study had some limitations. First, it was a single-institutional retrospective study. The sensitivity results may be biased because the reader knew that pathologically proven breast cancer existed in the presented PET images. Second, because the scan start time of DbPET was later than that of WBPET, the tumor FDG uptake of DbPET may be higher than that of WBPET. This gave DbPET an advantage over WBPET. Third, this study only compared the sensitivity of DbPET and WBPET and did not conduct a comparative analysis with other commonly used modalities, such as breast ultrasonography and breast MRI; prospective studies to compare all these modalities are required.
Conclusion
DbPET is an effective imaging modality for the detection of small and low-grade breast cancer and has potential utility for screening for multiple ipsilateral and bilateral cancerous lesions. Additionally, the SUVmax of DbPET could be used as an adjunct to immunohistochemistry for differentiating between molecular subtypes.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CT:
-
Computed tomography
- DbPET:
-
Dedicated breast positron emission tomography
- ER:
-
Estrogen receptor
- FDG:
-
18F-fluorodeoxyglucose
- FOV:
-
Field of view
- HER2:
-
Human epidermal growth factor receptor type 2
- LBR:
-
Lesion-to-background ratio
- MRI:
-
Magnetic resonance imaging
- PEM:
-
Positron emission mammography
- PET:
-
Positron emission tomography
- ROI:
-
Region of interest
- SUVmax:
-
Maximum standardized uptake value
- WBPET:
-
Whole body positron emission tomography
References
Welch HG, Prorok PC, O’Malley AJ, Kramer BS (2016) Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med 375:1438–1447
Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L (2016) Screening for breast cancer: a systematic review to update the 2009 U.S. preventive services task force recommendation. Ann Intern Med 164:244–255
Ichizawa N, Fukutomi T, Iwamoto E, Akashi-Tanaka S (2002) Long-term results of T1a, T1b and T1c invasive breast carcinomas in Japanese women: validation of the UICC T1 subgroup classification. Jpn J Clin Oncol 32:108–109
Escalona S, Blasco JA, Reza MM, Andradas E, Gómez N (2010) A systematic review of FDG-PET in breast cancer. Med Oncol 27:114–129
Ohara M, Shigematsu H, Tsutani Y, Emi A, Masumoto N, Ozaki S et al (2013) Role of FDG-PET/CT in evaluating surgical outcomes of operable breast cancer-usefulness for malignant grade of triple-negative breast cancer. Breast 22:958–963
Sasada S, Masumoto N, Suzuki E, Sueoka S, Goda N, Kajitani K et al (2019) Prediction of biological characteristics of breast cancer using dual-phase FDG PET/CT. Eur J Nucl Med Mol Imaging 46:831–837
Kadoya T, Aogi K, Kiyoto S, Masumoto N, Sugawara Y, Okada M (2013) Role of maximum standardized uptake value in fluorodeoxyglucose positron emission tomography/computed tomography predicts malignancy grade and prognosis of operable breast cancer: a multi-institute study. Breast Cancer Res Treat 141:269–275
Aogi K, Kadoya T, Sugawara Y, Kiyoto S, Shigematsu H, Masumoto N et al (2015) Utility of 18F FDG-PET/CT for predicting prognosis of luminal-type breast cancer. Breast Cancer Res Treat 150:209–217
Kumar R, Chauhan A, Zhuang H, Chandra P, Schnall M, Alavi A (2006) Clinicopathologic factors associated with false negative FDG-PET in primary breast cancer. Breast Cancer Res Treat 98:267–274
Kanumuri P, Hayse B, Killelea BK, Chagpar AB, Horowitz NR, Lannin DR (2015) Characteristics of multifocal and multicentric breast cancers. Ann Surg Oncol 22:2475–2482
Wolters R, Wöckel A, Janni W, Novopashenny I, Ebner F, Kreienberg R et al (2013) Comparing the outcome between multicentric and multifocal breast cancer: what is the impact on survival, and is there a role for guideline-adherent adjuvant therapy? A retrospective multicenter cohort study of 8,935 patients. Breast Cancer Res Treat 142:579–590
Lynch SP, Lei X, Chavez-MacGregor M, Hsu L, Meric-Bernstam F, Buchhholz TA et al (2012) Multifocality and multicentricity in breast cancer and survival outcomes. Ann Oncol 23:3063–3069
Yerushalmi R, Kennecke H, Woods R, Olivotto IA, Speers C, Gelmon KA (2009) Does multicentric/multifocal breast cancer differ from unifocal breast cancer? An analysis of survival and contralateral breast cancer incidence. Breast Cancer Res Treat 117:365–370
Ozturk A, Alco G, Sarsenov D, Ilgun S, Ordu C, Koksal U et al (2018) Synchronous and metachronous bilateral breast cancer: a long-term experience. J Boun 23:1591–1600
Michowitz M, Noy S, Lazebnik N, Aladjem D (1985) Bilateral breast cancer. J Surg Oncol 30:109–112
Gogas J, Markopoulos C, Skandalakis P, Gogas H (1993) Bilateral breast cancer. Am Surg 59:733–735
Tafra L, Cheng Z, Uddo J, Lobrano MB, Stein W, Berg WA et al (2005) Pilot clinical trial of 18F-fluorodeoxyglucose positron-emission mammography in the surgical management of breast cancer. Am J Surg 190:628–632
Yamamoto Y, Ozawa Y, Kubouchi K, Nakamura S, Nakajima Y, Inoue T (2015) Comparative analysis of imaging sensitivity of positron emission mammography and whole-body PET in relation to tumor size. Clin Nucl Med 40:21–25
Berg WA, Weinberg IN, Narayanan D, Lobrano ME, Ross E, Amodei L et al (2006) High-resolution fluorodeoxyglucose positron emission tomography with compression (“positron emission mammography”) is highly accurate in depicting primary breast cancer. Breast J 12:309–323
Nishimatsu K, Nakamoto Y, Miyake K, Ishimori T, Kanao S, Toi M et al (2017) Higher breast cancer conspicuity on dbPET compared to WB-PET/CT. Eur J Radiol 90:138–145
Brierley JD, Gospodararowicz MK, Wittekind C (2016) Union for International Cancer Control (UICC) TNM classification of malignant tumours, 8th Edition. Oxford
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S et al (2010) American Society of Clinical Oncology/College of American Pathologists Guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784–2795
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M et al (2015) Tailoring therapies–improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol 26:1533–1546
Kumar R, Alavi A (2004) Fluorodeoxyglucose-PET in the management of breast cancer. Radiol Clin North Am 42:1113–1122
Hosono M, Saga T, Ito K, Kumita S, Sasaki M, Senda M et al (2014) Clinical practice guideline for dedicated breast PET. Ann Nucl Med 28:597–602
Sasada S, Masumoto N, Goda N, Kajitani K, Emi A, Kadoya T et al (2018) Which type of breast cancers is undetectable on ring-type dedicated breast PET? Clin Imaging 51:186–191
Sasada S, Masumoto N, Kimura Y, Emi A, Kadoya T, Okada M (2020) Classification of abnormal findings on ring-type dedicated breast PET for the detection of breast cancer. Anticancer Res 40:3491–3497
Duffy SW, Tabár L, Yen AM, Dean PB, Smith RA, Jonsson H et al (2020) Mammography screening reduces rates of advanced and fatal breast cancers: results in 549,091 women. Cancer 126:2971–2979
Moscoso A, Ruibal Á, Domínguez-Prado I, Fernández-Ferreiro A, Herranz M, Albaina L et al (2018) Texture analysis of high-resolution dedicated breast 18 F-FDG PET images correlates with immunohistochemical factors and subtype of breast cancer. Eur J Nucl Med Mol Imaging 45:196–206
Acknowledgements
We thank Kazushi Marukawa and Masatsugu Tsujimura of Chuden Hospital for providing data regarding PET examinations.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Institutional Review Board approved this study. All procedures performed involving human participants were in accordance with the ethical standards of the institutional research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this retrospective study, the need for formal consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sueoka, S., Sasada, S., Masumoto, N. et al. Performance of dedicated breast positron emission tomography in the detection of small and low-grade breast cancer. Breast Cancer Res Treat 187, 125–133 (2021). https://doi.org/10.1007/s10549-020-06088-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-06088-1