Abstract
Purpose
Neoadjuvant endocrine therapy (NET) has been shown to be effective in ER-positive/HER2-negative breast cancer in clinical trials. However, adoption in clinical practice is still limited. Real-world data may provide useful insights into effectiveness, toxicities and quality of care, potentially rendering clinical trial results to the real-world setting. Our purpose was to report real-world data of a cohort of postmenopausal patients submitted to NET.
Methods
This prospective cohort study evaluated 146 postmenopausal female patients with ER-positive/HER2-negative breast cancer treated with NET at three tertiary hospitals between 2016 and 2018. Clinicopathological information were collected prospectively. Preoperative Endocrine Prognostic Index (PEPI) score was calculated for tumors submitted to at least 16 weeks of NET.
Results
Median age was 67 years old, and 87.8% had stage I-II disease. Most tumors had histological grade II (76.1%). Median pretreatment Ki67 expression was 10%. Aromatase inhibitor was used in 99.5% of patients, and median treatment duration was 21.0 weeks. No tumor progressed during NET. Breast-conserving surgery was performed in the majority of patients (63.0%), as well as sentinel lymph-node biopsy (76.7%). Pathological complete response rate was 1.0%. 43 patients (29.5%) had PEPI score 0, and 26% had PEPI scores 4–5. Posttreatment Ki67 median expression was 3.0%, and only five tumors (3.4%) showed marked increase in Ki67 expression during treatment. Seven patients (4.8%) had HER2-positive residual disease, and were treated with adjuvant chemotherapy plus trastuzumab.
Conclusions
Our real-world data shows that NET is effective and safe in postmenopausal patients with ER-positive/HER2-negative breast cancer. Postmenopausal status and low-risk luminal tumor features (luminal A-like) should be used as selection criteria to ensure the best results with NET.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neoadjuvant endocrine therapy (NET) has gained renewed attention in the last 20 years. NET was historically used as an alternative to chemotherapy in older and frail patients with estrogen receptor (ER)-positive breast cancer [1]. However, we have accumulating evidence that NET is an effective and well tolerated alternative to postmenopausal patients with ER-positive/HER2-negative breast cancer, not only to downstage tumors and allow less-extensive surgery, but as a scientific platform to obtain information on tumor resistance and potential biomarkers [2,3,4,5].
Residual tumor burden after neoadjuvant therapy has been validated as an important prognostic and predictive tool after neoadjuvant chemotherapy [6, 7]. Pathological complete response (pCR) can predict long-term outcomes and the benefit of additional systemic therapy after surgery, especially in triple-negative and HER2-positive breast cancer [8,9,10,11]. In ER-positive/HER2-negative breast cancer, pCR is rare either with chemotherapy (6–8%) and endocrine therapy (1%), and an association with long-term outcomes appears to valid only for high-grade tumors submitted to neoadjuvant chemotherapy [3, 9, 12, 13]. In an attempt to identify predictors of long-term outcomes after NET, a clinical multiparametric tool was developed and validated [4, 14]. Preoperative endocrine prognostic index (PEPI) takes into consideration tumor size, lymph node involvement, ER expression, and Ki67 expression on surgical specimen after 4 to 6 months of NET. Tumors with lower scores are associated with excellent prognosis [4, 14].
Randomized controlled trials (RCTs) are an ideal design for evaluating the effects of medical interventions under strictly controlled conditions. Patient selection and randomization help reduce bias and guarantee internal validity. However, these highly selected populations may not represent the more heterogeneous characteristics of patients seen in clinical practice, jeopardizing the generalizability of the results (external validity). Frequently drugs do not show the same results in clinical practice as reported in clinical trials, and this disparity of findings has been termed “efficacy-effectiveness gap” [15]. Real-world data (RWD) is being increasingly used to bridge that gap, by regulatory agencies, healthcare providers and even pharmaceutical industries. This kind of data provides useful insights into treatment access, effectiveness, toxicities, as well as the quality of care, guiding quality improvement interventions [16,17,18]. There are no RWD regarding the use of NET in ER-positive/HER2-negative breast cancer.
The aim of this study was to analyze prospectively collected data from patients submitted to NET and report RWD on patient characteristics, treatment, and surgical outcomes in clinical practice.
Methods
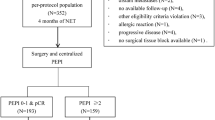
This was a prospective cohort study of luminal breast cancer patients treated with NET at three tertiary hospitals in Brazil (Women’s Hospital Prof. Dr. José Aristodemo Pinotti—Campinas/SP—CAISM, Pontifical Catholic University, PUC—Porto Alegre/RS—PUCRS, and Serra Gaúcha Research Center, Caxias do Sul/RS—CEPESG). Institutional Review Board approval of the study protocol was obtained in all participating institutions. Patients were included if they were postmenopausal with histological diagnosis of stage I-III ER-positive/HER2-negative breast cancer, and were submitted to NET, with treatment initiated between January 2016 and December 2018. We excluded patients with metastatic disease, who received neoadjuvant chemotherapy, refused or were unfit for surgery, and those lost to follow-up. We evaluated a total of 392 patients submitted to neoadjuvant therapy. Among them, 146 received NET and were included in the analysis. (Fig. 1) Since only postmenopausal patients were considered for NET, none of the patients received concomitant ovarian suppression.
Data were prospectively collected from patient charts, including age at diagnosis, clinicopathological variables, type and duration of treatment, type of breast and axillary surgery (sentinel lymph node biopsy, SLNB; or axillary lymph node dissection, ALND), and pathological specimen analyses. Pathological complete response was defined as absence of invasive disease in the breast and axilla (ypT0/is ypN0) [9]. Immunohistochemistry for ER (clone 1D5, 1:1.000, Dako) and PR (clone PR 636, 1:800, Dako) expression was done according to international consensus, and positivity was considered when at least 1% of the nuclei stained [19, 20]. HER2 expression was initially evaluated with immunohistochemistry (clone PN2A, 1:1.000), and staining was scored as 0 + /1 + (negative), 2 + (equivocal), and 3 + (positive). Equivocal cases were, then, confirmed by in situ hybridization, according to the ASCO/CAP recommendations [21]. Ki67 expression was conducted by immunohistochemistry (clone MIB1, 1:500, Dako) and defined as the mean expression in the whole tumor area [22]. Preoperative Endocrine Prognostic Index (PEPI) calculation were done as described by Ellis et al.[14] Clinical and pathological stage were defined according to the American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) TNM staging 8th edition [23].
We conducted descriptive analyses on the data collected for this study. Categorical variables were described as frequency and percentage, while continuous variables were reported as median. Data were collected and managed using REDCap (hosted by the State University of Campinas).
Results
Characteristics of the cohort of 146 patients are described in Table 1. Median age was 67 years old (51 to 93 years old), 73 (50%) had T2 tumors, and 128 (87.8%) were stage I-II disease. Ductal invasive carcinoma comprised 75.3% of tumors (n = 110), 111 (76.1%) were histological grade II, and median Ki67 expression were 10.0% (1–80%). ER positivity with Allred scores 3–8 were found in 134 patients (91.8%), and for PR in 114 patients (78.1%). Aromatase inhibitors (anastrozole or letrozole) were used by 99.5% of patients (n = 145), and median duration of treatment were 21.0 weeks (2 to 43 weeks). We did not observe clinical progression during treatment.
92 patients (63.0%) were submitted to breast conserving surgery, and sentinel lymph node biopsy (SLNB) were done in 112 patients (76.7%). Among patients in whom a SLNB was performed, 73 (50.0%) had a negative result. A positive SLNB was followed by axillary lymph node dissection (ALND) in 18 patients (12.3%). Upfront ALND was performed in 34 patients (23.3%) (Table 2).
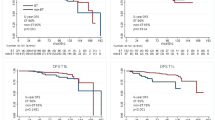
As shown in Table 3, pathological complete response (pCR) was achieved in only 2 patients (1.0%). 65 patients (44.5%) had residual pathological T2 tumors, and 82 patients (56.2%) were N0. Regarding PEPI score, 43 patients (29.5%) had score 0, and 38 (26.0%) had PEPI 4–5. 7 patients (4.8%) had HER2-positive disease at residual tumor specimen. Posttreatment Ki67 expression had a median value of 3.0% (1–80%). Figure 2 represents the change in Ki67 expression for each tumor before and after NET, showing that the majority of them had a decrease in Ki67 expression, and a marked increase in five cases (3.4%).
Detailed information regarding PEPI score individual components for the whole cohort are shown in Table 4. The majority of tumors were pathological T1/T2 (91.8%), and 40.4% had positive lymph nodes. Almost half of tumors (46.5%) had low levels of Ki67 expression (0–2,7%). All tumors with available information for hormone receptor expression were ER-positive.
Adjuvant chemotherapy was given to 26 patients (17.8%), and all patients (n = 7) with residual HER2-positive disease received HER2-targeted therapy. Adjuvant radiation therapy was performed in 102 patients (69.9%). (Table 5).
Discussion
Our study examined the use of NET in real-world setting, showing that it is a safe and effective neoadjuvant therapy in postmenopausal patients with ER-positive/HER2-negative breast cancer in clinical practice. Our analysis of real-world patients compared with phase 3 clinical trials of NET revealed a similar median age at treatment initiation, although slightly inferior than in IMPACT trial [3,4,5]. Half of our patients had T2 tumors, and most patients were classified as having stage I disease. Accordingly, in P024 trial approximately half of patients had T2N0 disease, and median tumor size were 3.6 cm in PROACT trial, and 3.8–4.0 cm in IMPACT trial [3,4,5]. A tumor size of at least 3.0 cm was required for enrollment in P024 and PROACT trials, but one third of our cohort patients had tumors of 2.0 cm or less [3, 5]. In our clinical practice, we offered NET event to patients with small, operable tumors while we wait for surgery, due to long waiting times, especially in the first months after inclusion of NET in our clinical protocols. Also, older patients initially deemed unfit for surgery went on to surgical management after a period of NET.
Tumor proliferation as measured by Ki67 expression was markedly reduced in the majority of our patients. Posttreatment Ki67 median expression was 70% lower than pretreatment value, and virtually all patients were treated with an aromatase inhibitor. Endocrine therapy induces cell-cycle arrest, and evaluation of tumor proliferation may be a surrogate of treatment effect [24]. Indeed, data from P024 trial showed that Ki67 expression in surgical specimen was associated with disease-free survival and breast cancer specific survival [14]. Mean reduction in Ki67 expression with aromatase inhibitors were of 87% in P024, 81.6% in IMPACT, and 78% in ACOSOG Z1031 [12, 14, 25]. Although our median pretreatment Ki67 expression was low, we had some patients with higher Ki67 expression, probably because of ineligibility for chemotherapy. Four tumors had a marked increase in Ki67 expression, and two of them revealed HER2-overexpression at surgical specimen.
The optimal duration of NET was not defined. In our cohort, treatment duration varied from 2 to 43 weeks, with a median time of 21 weeks. Shorter treatment periods occurred mostly during the first months of protocol implementation and is part of the learning curve. Our goal is to maintain NET for at least 16 weeks, as recommended for PEPI score calculation. In clinical trials of NET, treatment was recommended for a period of 3–4 months.[3,4,5] However, several studies have suggested that a longer duration of treatment are required for maximum effect, and approximately one third of patients achieve maximal response after 6 months.[26,27,28,29] Our median NET duration approaches 6 months and, although longer therapy duration may be associated with the development of resistance, none of our patients progressed during treatment.
The majority of patients in our cohort were submitted to breast-conserving surgery. We did not collect data on surgery conversion rates, but our rate is somewhat higher than was reported for AI in clinical trials [3,4,5]. This may be explained by the inclusion of a large proportion of T1 tumors and the longer treatment duration in our cohort. Trials that compared NET to neoadjuvant chemotherapy reported breast-conserving surgery rates of 24 to 54.6% with chemotherapy [30,31,32]. For example, NEOCENT trial compared neoadjuvant letrozole to anthracycline-based chemotherapy, reporting breast-conserving surgery rates of 68.2% vs 54.6%, respectively [32]. Most patients in our cohort were submitted to SLNB, and less than one fourth had a positive sentinel node. In ACOSOG Z1031 the rate of SLNB ranged from 31.4 to 61.3% in tumors with a week 2 Ki67 > 10% or ≤ 10%, respectively.
Our pCR rate is consistent with previous studies on NET [3]. We observed a PEPI 0 rate of 29.5% and PEPI 1 of 17.0%. In ACOSOG Z1031, approximately 17% of patients had PEPI 0 [12]. This difference could be due to the higher proportion of luminal A-like tumors in our cohort. Endocrine therapy-resistant disease (PEPI 4–5) comprised 26% of our patients, and some of these patients (39%) received adjuvant chemotherapy, because of the perceived high risk of recurrence and mortality in these group of patients. None of our patients with PEPI 0 received adjuvant chemotherapy, in contrast to 11% in ACOSOG Z1031. On the other hand, 25.4% of our PEPI > 0 patients received adjuvant chemotherapy, in comparison to 41.2% in ACOSOG Z1031 [33]. This reflects the fact that the best approach after NET is still undefined. This issue is being evaluated in ALTERNATE trial (NCT01953588) [34]. A small subset of patients with low PEPI scores were submitted to adjuvant chemotherapy due to HER2-overexpression in surgical specimens. The rate of conversion to HER2-positive disease was of 4.8%, in accordance with what was previously published with neoadjuvant chemotherapy [35].
The strengths of our study include the inclusion of a more diverse group of patients, when compared to randomized clinical trials, and the fact that it reflects real-world treatment patterns, outcomes and decision-making process. Our study is limited by its nonrandomized nature, and the short-term follow-up. However, patients are still being followed and data on long-term outcomes will be available in the future. To the best of our knowledge this is the first prospective cohort to report on patients submitted to NET outside of a clinical trial.
Furthermore, one potential concern regarding NET is the risk of disease progression, in part due to the cytostatic, and not cytotoxic, effect of these agents [24]. Several translational studies have sought to evaluate potential biomarkers to identify patients who would show better responses to NET and those with primary resistance, such as the incorporation of algorithms based on the early effect on tumor proliferation (Ki67 expression after 2–4 weeks of treatment) and genomic profiling as in the 21-gene recurrence score assay (Oncotype DX) [34, 36, 37]. It is noteworthy that none of the patients in our real-world cohort had disease progression during NET, even though they were selected based solely on traditional clinical and pathological data, without the addition of modern molecular biology data. Our data highlights the ideal patient profile for NET (postmenopausal patients, tumors with luminal A-like features), and suggests that progression during NET is probably associated with its use in non-ideal patients (eg, luminal B-like tumors, and premenopausal patients). These findings are clinically relevant and should provide support for the adoption on NET in the clinical practice.
In conclusion, our prospective real-world data on postmenopausal patients with ER-positive/HER2-negative breast cancer shows that NET is a safe and effective alternative to chemotherapy, with similar outcomes than what was reported in the literature. These results may help increase awareness and adoption of NET in clinical practice.
Data availability
All the authors had full access to all the data in the study and vouch for the decision to submit the manuscript for publication.
References
Chia YH, Ellis MJ, Ma CX (2010) Neoadjuvant endocrine therapy in primary breast cancer: indications and use as a research tool. Brit J Cancer 103(6):759–764
Reinert T, Ramalho S, Gonçalves R, Barrios CH, Graudenz MS, Bines J (2016) Multidisciplinary approach to neoadjuvant endocrine therapy in breast cancer: a comprehensive review. Rev Bras Ginecol Obstet Rev Bras Soc Ginecol Obstet 38(12):615–622
Eiermann W, Paepke S, Appfelstaedt J, Llombart-Cussac A, Eremin J, Vinholes J et al (2001) Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol 12(11):1527–1532
Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer J-U et al (2005) Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 23(22):5108–5116
Cataliotti L, Buzdar AU, Noguchi S, Bines J, Takatsuka Y, Petrakova K et al (2006) Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the pre-operative “Arimidex” compared to Tamoxifen (PROACT) trial. Cancer 106(10):2095–2103
Sheri A, Smith IE, Johnston SR, A’Hern R, Nerurkar A, Jones RL et al (2015) Residual proliferative cancer burden to predict long-term outcome following neoadjuvant chemotherapy. Ann Oncol Off J Eur Soc Med Oncol 26(1):75–80
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V et al (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25(28):4414–4422
Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL et al (2020) Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res 26(12):2838–2848
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172
Masuda N, Lee S-J, Ohtani S, Im Y-H, Lee E-S, Yokota I et al (2017) Adjuvant capecitabine for breast cancer after preoperative chemotherapy. New Engl J Med 376(22):2147–2159
von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M et al (2019) Trastuzumab emtansine for residual invasive HER2-positive breast cancer. New Engl J Med 380(7):617–628
Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A et al (2011) Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol Off J Am Soc Clin Oncol 29(17):2342–2349
Gonzalez-Angulo AM, Iwamoto T, Liu S, Chen H, Do K-A, Hortobagyi GN et al (2012) Gene expression, molecular class changes, and pathway analysis after neoadjuvant systemic therapy for breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res 18(4):1109–1119
Ellis MJ, Tao Y, Luo J, A’Hern R, Evans DB, Bhatnagar AS et al (2008) Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer I 100(19):1380–1388
Eichler H-G, Abadie E, Breckenridge A, Flamion B, Gustafsson LL, Leufkens H et al (2011) Bridging the efficacy-effectiveness gap: a regulator’s perspective on addressing variability of drug response. Nat Rev Drug Discov 10(7):495–506
Booth CM, Karim S, Mackillop WJ (2019) Real-world data: towards achieving the achievable in cancer care. Nat Rev Clin Oncol 16(5):312–325
Katkade VB, Sanders KN, Zou KH (2018) Real world data: an opportunity to supplement existing evidence for the use of long-established medicines in health care decision making. J Multidiscip Healthc 11:295–304
Sherman RE, Anderson SA, Pan GJD, Gray GW, Gross T, Hunter NL et al (2016) Real-world evidence—what is it and what can it tell us? New Engl J Med 375(23):2293–2297
Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S et al (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 28(16):2784–2795
Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL et al (2020) Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol Off J Am Soc Clin Oncol. https://doi.org/10.1200/JCO.19.02309
Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice guideline update. J Clin Oncol 31(31):3997–4013
Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J et al (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer I 103(22):1656–1664
Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ et al (2017) Breast cancer-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. Ca Cancer J Clin 67(4):290–303
Goncalves R, Ma C, Luo J, Suman V, Ellis MJ (2012) Use of neoadjuvant data to design adjuvant endocrine therapy trials for breast cancer. Nat Rev Clin Oncol 9(4):223–229
Dowsett M, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I et al (2005) Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer—a study from the IMPACT trialists. J Clin Oncol 23(11):2477–2492
Dixon JM, Renshaw L, Macaskill EJ, Young O, Murray J, Cameron D et al (2008) Increase in response rate by prolonged treatment with neoadjuvant letrozole. Breast Cancer Res Tr 113(1):145–151
Fontein DBY, Charehbili A, Nortier JWR, Kranenbarg EM-K, Kroep JR, Putter H et al (2014) Efficacy of six month neoadjuvant endocrine therapy in postmenopausal, hormone receptor-positive breast cancer patients—a phase II trial. Eur J Cancer. 50(13):2190–2200
Krainick-Strobel UE, Lichtenegger W, Wallwiener D, Tulusan AH, Jänicke F, Bastert G et al (2008) Neoadjuvant letrozole in postmenopausal estrogen and/or progesterone receptor positive breast cancer: a phase IIb/III trial to investigate optimal duration of preoperative endocrine therapy. BMC Cancer 8(1):62
Carpenter R, Doughty JC, Cordiner C, Moss N, Gandhi A, Wilson C et al (2014) Optimum duration of neoadjuvant letrozole to permit breast conserving surgery. Breast Cancer Res Tr 144(3):569–576
Semiglazov VF, Semiglazov VV, Dashyan GA, Ziltsova EK, Ivanov VG, Bozhok AA et al (2007) Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer 110(2):244–254
Alba E, Calvo L, Albanell J, la Haba JRD, Lanza AA, Chacon JI et al (2012) Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol Off J Eur Soc Med Oncol 23(12):3069–3074
Palmieri C, Cleator S, Kilburn LS, Kim SB, Ahn S-H, Beresford M et al (2014) NEOCENT: a randomised feasibility and translational study comparing neoadjuvant endocrine therapy with chemotherapy in ER-rich postmenopausal primary breast cancer. Breast Cancer Res Tr 148(3):581–590
Ellis MJ, Suman VJ, Hoog J, Goncalves R, Sanati S, Creighton CJ et al (2017) Ki67 Proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J Clin Oncol 35(10):1061–1069
Suman VJ, Ellis MJ, Ma CX (2015) The ALTERNATE trial: assessing a biomarker driven strategy for the treatment of post-menopausal women with ER+/Her2- invasive breast cancer. Chin Clin Oncol 4(3):34
Niikura N, Tomotaki A, Miyata H, Iwamoto T, Kawai M, Anan K et al (2015) Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese breast cancer registry. Ann Oncol Off J Eur Soc Med Oncol Esmo 27(3):480–487
Dowsett M, Ellis MJ, Dixon JM, Gluz O, Robertson J, Kates R et al (2020) Evidence-based guidelines for managing patients with primary ER+ HER2- breast cancer deferred from surgery due to the COVID-19 pandemic. NPJ Breast Cancer 6(1):21
Iwata H, Masuda N, Yamamoto Y, Fujisawa T, Toyama T, Kashiwaba M et al (2018) Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, HER2-negative breast cancer: the TransNEOS study. Breast Cancer Res Tr 173(1):123–133
Funding
Silva LR—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Brasil Finance Code 001. The funding sources did not have any direct involvement on the collection, analysis, and interpretation of the data, nor did they were involved on the writing and revision of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by LRS, CAA, SR, TR, ABAS, AERS, MBPLK, VCAV. Analysis was performed by LRS, CAA, FB, SR. The first draft of the manuscript was written by LRS, CAA, FB, SR. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Leonardo Roberto da Silva and Camila Annicchino de Andrade are co-first authors.
Rights and permissions
About this article
Cite this article
da Silva, L.R., de Andrade, C.A., Brenelli, F. et al. Real-world data on neoadjuvant endocrine therapy in ER-positive/HER2-negative breast cancer. Breast Cancer Res Treat 186, 753–760 (2021). https://doi.org/10.1007/s10549-020-06076-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-06076-5