Abstract
Purpose
Recent evidence supports the efficacy of scalp cooling in preventing chemotherapy-induced alopecia in breast cancer treatments. However, efficacy largely varies between treatment regimens. The aim of this study was to explore the patient- and nurse-reported results of scalp cooling in terms of hair loss and need for a wig/head cover in patients with breast cancer treated with 3-weekly docetaxel 75 mg/m2– cyclophosphamide 600 mg/m2.
Methods
We studied nurse-reported efficacy as noted in the electronic patient files of 85 patients treated with docetaxel 75 mg/m2– cyclophosphamide 600 mg/m2 between 1/1/2017 and 1/1/2020. Sixty-nine of them also self-reported on their scalp cooling results up to one year after adjuvant chemotherapy in a retrospective way.
Results
Nurse- and patient-reported data showed that scalp cooling was successful (i.e., hair loss < 50%) in 47.1 and 44.9% of patients, respectively, and 55% of patients were (very) satisfied with the result of scalp cooling. Scalp cooling was perceived as (very) uncomfortable in 36.2% of patients. Regarding hair status one year after treatment, 47 patients (55.3%) reported no changes compared to their hair status before treatment.
Conclusions
Scalp cooling is successful in preventing severe chemotherapy-induced alopecia in almost half of the patients with breast cancer treated with docetaxel 75 mg/m2– cyclophosphamide 600 mg/m2. Better understanding of the success rate of scalp cooling enables correct patient information and decision-making support.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Hair loss is a tremendously difficult side-effect of cancer treatment for many patients. It alters patients’ self-image, negatively affects their self-esteem, and causes emotional distress [1,2,3,4]. For decades now, scalp cooling has been proposed for the prevention of chemotherapy-induced alopecia. However, due to costs, workload for hospital personnel, limited or weak evidence on its efficacy, and some early concerns about safety, specifically the potential for scalp metastases, it has only slowly been adopted in clinical cancer care.
Recent evidence is turning the tide for scalp cooling. A recent review and meta-analysis of 24 longitudinal studies in breast cancer showed that scalp cooling didn’t increase the risk for scalp metastases [5]. Furthermore, recent studies add to the evidence supporting the efficacy and safety of scalp cooling in preventing chemotherapy-induced alopecia [6,7,8]. A non-randomized cohort study with anthracycline-free chemotherapy regimens in breast cancer, demonstrated that 66% of patients in the scalp cooling group had hair loss of 50% or less, compared to 0% in the group without scalp cooling [7]. The COOLHAIR study, a randomized controlled trial using breast cancer chemotherapy regimens with both anthracyclines and taxanes, showed hair preservation of 39.3% in patients with scalp cooling versus 0% in the control group (p < 0.001) [8]. Additionally, the outside use of a head cover was significantly lower in the scalp cooling group (40.7% versus 95.5%, p < 0.001). The SCALP trial studied scalp cooling in the same target group (anthracyclines + taxanes) and showed hair preservation in 50.5% of patients with scalp cooling, compared to 0% in the control group [6]. Finally, an observational study by Gianotti et al. showed hair preservation in 89% of women receiving taxane-based chemotherapy, in 78% of women receiving both anthracyclines and taxanes and in 47% of women undergoing anthracycline-based chemotherapy [9].
Available evidence suggests that scalp cooling is more effective for taxane-based chemotherapy regimens when compared to anthracycline-taxane-based chemotherapy regimens [9, 10]. However, mixed regimens in many of the studies hinder the extraction of efficacy data for specific regimens.
Scalp cooling has been reintroduced in our hospital in 2014, aiming to offer scalp cooling to patients treated with weekly paclitaxel ≤ 100 mg/m2 or three-weekly docetaxel ≤ 75 mg/m2, unless these are (even sequentially) combined with anthracyclines. Based on our experiences in daily practice, we noticed a big difference in the success rate of scalp cooling between regimens, in disadvantage of the patients treated with docetaxel–cyclophosphamide. First, we examined and altered post-cooling times. While we had applied short post-cooling times of 20′ and 45′ based on evidence on post-cooling time in docetaxel [11, 12], we soon expanded post-cooling to 90′ after the infusion of cyclophosphamide to improve our success rates.
The aim of this study was to explore the patient- and nurse-reported results of scalp cooling in terms of hair loss and need for a wig/head cover in patients with breast cancer treated with 3-weekly Docetaxel 75 mg/m2– Cyclophosphamide 600 mg/m2 (Table 1).
Methods
Design
This observational study comprises two parts, both targeting patients with breast cancer who had used scalp cooling to prevent alopecia associated with docetaxel–cyclophosphamide. The first part was a retrospective evaluation on the basis of nurse-reported scalp cooling results in the Electronic Patient Records (EPR). The second part of the study used a prospective descriptive design with surveys to explore the patient-reported results of scalp cooling. Additionally, we explored the agreement between nurse- and patient-reported results of scalp cooling.
Setting
The study took place at the Multidisciplinary Breast Centre of the University Hospitals Leuven, Belgium, where scalp cooling is applied for breast cancer patients treated with taxanes (without anthracyclines).
Sample
The study included adult (≥ 18 years) patients with breast cancer who.
-
1.
had been treated with 3-weekly docetaxel 75 mg/m2– cyclophosphamide 600 mg/m2 for four or six cycles in (neo-)adjuvant setting between 1/1/2017 and 1/1/2020
-
2.
had received scalp cooling to prevent chemotherapy-induced alopecia with 90 min post-infusion cooling time after the infusion of cyclophosphamide
Patients were included in part 2 of the study if they had signed an informed consent for this study.
Scalp cooling procedure
Scalp cooling was administered by the nurses working at the oncology daycare center. Paxman-cooling devices (both Paxman-Orbis and PSCS Paxman-Orbis New Style cooling machines) were used. Scalp cooling was initiated 30 min prior to each docetaxel (which was administered first) and was continued for 90 min after the administration of cyclophosphamide. Scalp cooling was free of charge to patients.
Study procedure
For both parts of the study, we started from a list of patients in our hospital, treated with docetaxel 75 mg/m2– cyclophosphamide 600 mg/m2 between 1/1/2017 and 1/1/2020, as derived from the EPR software system based on the electronic chemotherapy prescription for this regimen.
All data for part 1 of the study were derived from the EPR. For the second part of the study, all included patients in part 1 were sent written information about the study and were given the opportunity to contact the researcher about their decline or their need for further oral information. If they agreed, they could instantly sign their informed consent, complete the survey and return it to the researchers. For patients who had not returned their questionnaire after the first sending, the researchers contacted the patient by telephone to offer oral information about the study and invited them to complete and return the survey as well as their informed consent. If the patient indicated difficulty in completing or returning the survey on paper, the researcher completed the survey on the basis of a telephone interview.
Data collection
At the time of the study, nurses evaluated and documented scalp cooling tolerability and results at each scalp cooling session as part of their standard care. Therefore, all data for the first part of the study (i.e., nurse-reported alopecia, need to wear a wig/head cover inside and outside the home, scalp cooling tolerability) were extracted from the EPR. Nurse-reported alopecia was graded based on 4 grades, as available in the EPR: no hair loss, thinning of the hair, hair loss of < 50%, hair loss of ≥ 50%. The need to wear a wig is reported on a 3-point Likert scale: never, sometimes, always. Tolerability was reported as the presence or absence of adverse effects (e.g. headaches) related to scalp cooling. Since nurses reported on scalp cooling results and tolerability as part of their daily nursing care, missing registration was taken into account by describing the reporting grade for both tolerability and results, i.e., the number of time points nurses had registered tolerability and results in proportion to the total number of scalp cooling sessions.
Patient-reported outcomes of scalp cooling comprised:
-
Patient-reported alopecia at the end of scalp cooling
-
Need to wear a wig/head cover inside and outside the home
-
Satisfaction with the result of scalp cooling
-
Level of comfort of both the cold temperature and the mobility restriction associated with scalp cooling
Patient-reported alopecia and need to wear a wig/head cover were graded in the same way as their nurse-reported match. Given the retrospective evaluation, patients could indicate if they didn’t remember their hair status well enough. Satisfaction and comfort were evaluated using a 5-point Likert scale (0 = very unsatisfied/uncomfortable, 5 = very satisfied/comfortable).
Patients who had finished their treatment (more than) one year ago, were additionally asked to report:
-
Patient-reported alopecia at 1 year after the end of treatment
-
Satisfaction with the current hair status
-
The extent to which they would recommend scalp cooling to others, assessed using a 5-point Likert scale (0 = absolutely not, 5 = absolutely yes)
Data analysis
We used IBM Statistical Package (SPSS v19) to perform data analysis.
Nurse- and patient-reported data were analyzed using descriptive statistics. Scalp cooling was considered as successful if patients reported no hair loss, just hair thinning or < 50% hair loss. If the patient reported hair loss of ≥ 50%, scalp cooling was considered to have failed. Based on the nurse-reported data, scalp cooling was considered to have failed when nurse reports indicated hair loss of ≥ 50% at least once during all scalp cooling sessions or when scalp cooling was prematurely interrupted.
Kappa value was calculated as a measure of agreement between patient- and nurse-reported success or failure of scalp cooling. A kappa value less than 0.4 suggests poor agreement, 0.4–0.75 implies fair-to-good agreement, and 0.75 or more suggests excellent agreement. Additionally, McNemar's exact test was performed based on the dichotomized nurse- and patient-reported results of scalp cooling (successful versus failed), with p < 0.05 indicating a significant systematic difference between nurse- and patient-reported results of scalp cooling.
Ethics
The study protocol was approved by the Ethics Committee of UZ/KU Leuven. For part 2 of the study, all respondents of the survey gave their informed consent. The dataset only contained pseudonymized data. Only the researchers had access to respondents’ identity and individual code.
Results
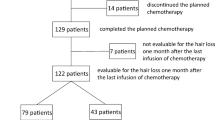
Eighty-five patients met the inclusion criteria for this study and file analysis of nurse-reported scalp cooling results was performed in all. All were invited to participate in the patient-reported evaluation of scalp cooling results and 69 (81.2%) patients did. Of these, 67 patients who had finished their treatment at least one year before, also reported on the scalp cooling results one year after treatment. The majority (70.6%) of patients was scheduled for six cycles of chemotherapy. All patients completed the planned number of cycles and dose was reduced in 9 patients (10.6%). Finally, 21 patients (24.7%) ended scalp cooling prematurely. Nurse-reported reasons for stopping scalp cooling were: severe hair loss (n = 15), poor tolerability (n = 1), combination of bad result and poor tolerability (n = 4), and not reported (n = 1).
Grade of alopecia was reported by the nurse in nearly 70% of scalp cooling sessions. Based on the nurse-reported results, 40 patients (47.1%) had lost less than 50% of their hair, indicating that scalp cooling was successful. According to the nurse evaluation, nine (10.6%) and eight (9.4%) patients had no hair change at all or just thinned hair, respectively.
Patient-reported data (Table 2) show that scalp cooling was successful in 31 (44.9%) of the patients, while 38 (55.1%) had lost 50% or more of their hair. Around half of the patients (53.6%) never used a head cover at home and 31.9% never did outside of the house. More than half of the patients (55.0%) were (very) satisfied with their scalp cooling results.
Regarding their hair status one year after treatment, 47 patients (55.3%) reported no changes compared to their hair status before treatment, while 15 (22.4%) reported to have thinned hair. Also, four patients (6.0%) reported to have still lost 50% or more of their hair when compared to their hair before treatment. One year after treatment, the same number of patients (i.e., 32 patients or 47.8%) reported to be (very) satisfied or either (very) dissatisfied with the scalp cooling results. Likewise, 40.3% of patients said they would absolutely recommend scalp cooling to other patients, while almost the same percentage said they would absolutely not.
Because of the small number of patients with ≥ 50% hair loss at one year after treatment (n = 4), we didn’t perform any statistical exploration of factors that might explain differences in hair status one year after treatment. Three of these four patients reported ≥ 50% hair loss during treatment, while one had reported less than 50% hair loss at that time. All four cases concerned patients who stopped scalp cooling early. Three of them had post-menopausal status, one patient was premenopausal. Finally, all four patients were having antihormonal treatment (three with letrozole, one with tamoxifen) at this time point. None of them had any other anticancer treatment since their treatment with docetaxel–cyclophosphamide.
Information on the absence or presence of side-effects related to scalp cooling was reported by the nurses in 70% of all scalp cooling sessions. Nurses had reported side-effects related to scalp cooling in 8 patients (9.4%). Based on the patient-reported data, 25 patients (36.2%) had experienced the cooling of the scalp as (very) uncomfortable, while 30 (43.5%) did not label this as comfortable nor uncomfortable. Regarding the mobility restriction associated with scalp cooling, 23 patients (33.3%) rated this as (very) uncomfortable, while 35 (50.7%) were neutral.
In free-text fields, some patients elaborated on their (dis)satisfaction with scalp cooling. Most frequent negative comments were: (1) suboptimal fit of the cold cap with hair loss on the crown of the head and (2) disappointment and emotional burden of failed scalp cooling. Some patients mentioned the challenge of bearing the cold. Most frequent positive comments concerned: (1) impact of successful scalp cooling on self-esteem and facing the outside world, (2) hair growing back faster than expected without scalp cooling after chemotherapy, and (3) gratitude for getting the offer of scalp cooling and the hope of keeping one’s hair. Finally, several patients commented about the need for correct information on the results of scalp cooling before the start of treatment, saying it should be clear that scalp cooling might fail and correct information on the success rate of scalp cooling is important for patients’ decision-making.
We used the kappa coefficient to assess the inter-rater reliability of agreement. With a kappa value of 0.352 (Table 3), we observed rather poor agreement between patient- and nurse-reported success or failure of scalp cooling. In 17.4% of cases, scalp cooling was successful on the basis of patient-reported data, while it had failed on the basis of nurse reports. At the same time, in 14.5% of the cases, scalp cooling had failed according to the patient report, while nurse reports indicated successful scalp cooling. The McNemar test was not statistically significant (p = 0.832, see Table 3) indicating no systematic difference between patient- and nurse-reported results.
Discussion
Treatment with 3-weekly docetaxel 75 mg/m2– cyclophosphamide 600 mg/m2 is a frequently used chemotherapy regimen in early breast cancer, mainly for the hormone receptor positive/HER2-negative subgroup. Given the burden and fear associated with treatment-related hair loss [13], scalp cooling offers a promising way for the prevention of alopecia. In this study, we evaluated the success of scalp cooling in 3-weekly docetaxel 75 mg/m2– cyclophosphamide 600 mg/m2 from the nurse- and patient perspective.
Scalp cooling was successful in preventing severe alopecia (i.e., ≥ 50% hair loss) in 44.9% of patients in our study. Of all patients with hair preservation, 64.5% had < 50% hair loss, 25.8% of them only had hair thinning and 9.7% reported no change to hair status. More than half of patients (55%) reported to be (very) satisfied with the results of scalp cooling at the time of their treatment and 29% were not. When looking back to the scalp cooling results one year after treatment, there were as many satisfied as dissatisfied patients (47.8%). One year after treatment, half of patients (50.7%) would recommend scalp cooling to other patients, whereas 41.8% wouldn’t. Remarkably, one year after treatment, patient-reported results seemed to have shifted to the extremes on both sides. While nurses reported side-effects related to scalp cooling in only 9.4% of the patients, 36.2% of patients rated the cold during scalp cooling as (very) uncomfortable, while 43.5% expressed no opinion.
It is difficult to compare our findings to those of other studies as these often comprise a variety of chemotherapy regimens and often don’t present regimen-specific results. In a prospective cohort study on scalp cooling in non-anthracycline-based chemotherapy for breast cancer, 75% of patients were treated with docetaxel 75 mg/m2–cyclophosphamide 600 mg/m2 and scalp cooling was successful (i.e., patient-reported Dean score < 3) in 60.5% of these patients, compared to 0% in the (small) control group [7]. This is substantially more than the 44.9% patient-reported success rate in our study. While patient and hair characteristics (e.g., hair thickness, liver function) as well as quality of the procedure (e.g., fit of the cap) and staff experience are known to substantially influence scalp cooling results [6, 14], these data are unavailable, and therefore, we cannot fully explain the differences between Rugo’s and our results. Rugo et al. reported post-infusion cooling times of 90–120 min after chemotherapy [7], which is in line with the fixed post-infusion cooling time of 90′ after cyclophosphamide infusion in our study. Extended post-cooling duration has been studied to improve the success rates of scalp cooling in anthracyclines [15] Of course, longer post-infusion cooling times impact patients’ stay in the hospital, associated nursing time and patient flow in the chemotherapy unit.
When using the results of this study to inform future patients and support them in their decision-making about scalp cooling, as several patients suggested, the results of this study confront them with a difficult dilemma. To address the information need in patients as well as healthcare professionals informing patients about this choice, Van den Hurk et al. developed a website and web-based decision tool to offer information on the probability of chemotherapy-induced alopecia with and without scalp cooling in particular chemotherapy regimens and to facilitate reflection on their values and opinions about scalp cooling [16]. Our study doesn’t present longitudinal data on the evolution of hair loss throughout chemotherapy cycles. Bitto et al. reported that, in the case of unsuccessful scalp cooling, hair loss generally occurred during the first two cycles of treatment [13]. The authors therefore suggested to stop scalp cooling after two cycles of treatment if the results are unfavorable. The question remains how failed scalp cooling should then be defined, as some patients are still satisfied with substantial hair loss or appreciate that their hair may grow back faster after treatment. This also raises the matter of late or permanent alopecia associated with docetaxel, as has been described in case reports [17]. One year after their treatment, four out of 67 patients (6%) in our study still reported to have lost more than 50% of their hair compared to before treatment. It is unclear to what extent this is related to the docetaxel–cyclophosphamide treatment, or to other factors (e.g., antihormonal therapy; adjuvant CDK4/6 inhibition, in clinical trial during the study period; or spontaneous alopecia occurring in menopause) that may contribute to hair thinning after breast cancer treatment. Given the relative small sample size in this study, the impact of these factors should be studied in further research. Permanent chemotherapy-induced alopecia has mainly been associated with taxane- and cyclophosphamide-based regimens [18]. Permanent chemotherapy-induced alopecia may present itself as diffuse alopecia or as androgenetic alopecia at the frontoparietal hairline [19]. Martin and colleagues found that persistent alopecia occurred in 10% of patients who had a cumulative dose of docetaxel ≥ 400 mg/m2 [20]. In contrast to the results of our study, the introduction of scalp cooling produced complete prevention of persistent alopecia in this patient group [20]. Extended follow-up in scalp cooling studies and comparison in patients without chemotherapy could clarify its efficacy in preventing permanent chemotherapy-related alopecia.
Regarding the agreement between patient- and nurse-reported results, a kappa of 0.352 indicated rather poor agreement in success or failure on the basis of patient-reported retrospective evaluation and nurse reports during treatment. This discordance is in contrast with the finding of Smetanay et al. [8] showing near complete concordance between patient and staff evaluation during the course of treatment [8]. It should be acknowledged, however, that in the Smetanay study, the evaluation was done on the same day by patients and professionals, while in our study, there was up to three years between nurse evaluation and patient evaluation, limiting the adequacy of this comparison. The McNemar test didn’t show any systematic difference between patients and nurses judgements; patients were not systematically more positive or negative compared to nurses.
Our study has several limitations. First, this was an observational study without any control group. Next, patients retrospectively reported on their scalp cooling results. While no patient indicated having difficulties remembering the severity of hair loss, recall bias can’t be ruled out completely. Nurses reported scalp cooling results as part of their daily clinical care and without clear protocol or training for evaluating the severity of alopecia. This may impact the reliability of the nurse-reported data.
Conclusion
This observational study aimed at exploring scalp cooling efficacy for preventing alopecia associated with docetaxel 75 mg/m2– cyclophosphamide 600 mg/m2 in early breast cancer. Patient-reported severe alopecia (≥ 50% hair loss) was prevented in 44.9% of the patients in our study. More than half of patients (55%) reported to be (very) satisfied with the results of scalp cooling at the time of their treatment. One year after treatment, there were as many satisfied as dissatisfied patients (47.8%). More than one out of three patients (36.2%) rated the cold during scalp cooling as (very) uncomfortable. These results can optimize patient counseling and shared decision-making for scalp cooling with docetaxel–cyclophosphamide.
Data availability
Data are available upon request via 10.5281/zenodo.4288685. Code is available upon reasonable request to the corresponding author.
References
Choi EK, Kim IR, Chang O, Kang D, Nam SJ, Lee JE, Lee SK, Im YH, Park YH, Yang JH et al (2014) Impact of chemotherapy-induced alopecia distress on body image, psychosocial well-being, and depression in breast cancer patients. Psychooncology 23(10):1103–1110
Frith H, Harcourt D, Fussell A (2007) Anticipating an altered appearance: women undergoing chemotherapy treatment for breast cancer. Eur J Oncol Nurs 11(5):385–391
Harcourt D, Frith H (2008) Women’s experiences of an altered appearance during chemotherapy: an indication of cancer status. J Health Psychol 13(5):597–606
Shaw J, Baylock B, O’Reilly A, Winstanley J, Pugliano L, Andrews K, Boyle F (2016) Scalp cooling: a qualitative study to assess the perceptions and experiences of Australian patients with breast cancer. Support Care Cancer 24(9):3813–3820
Rugo HS, Melin SA, Voigt J (2017) Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat 163(2):199–205
Nangia J, Wang T, Osborne C, Niravath P, Otte K, Papish S, Holmes F, Abraham J, Lacouture M, Courtright J et al (2017) Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA 317(6):596–605
Rugo HS, Klein P, Melin SA, Hurvitz SA, Melisko ME, Moore A, Park G, Mitchel J, Bageman E, D’Agostino RB Jr et al (2017) Association between use of a scalp cooling device and alopecia after chemotherapy for breast cancer. JAMA 317(6):606–614
Smetanay K, Junio P, Feisst M, Seitz J, Hassel JC, Mayer L, Matthies LM, Schumann A, Hennigs A, Heil J et al (2019) COOLHAIR: a prospective randomized trial to investigate the efficacy and tolerability of scalp cooling in patients undergoing (neo)adjuvant chemotherapy for early breast cancer. Breast Cancer Res Treat 173(1):135–143
Gianotti E, Razzini G, Bini M, Crivellaro C, Righi A, Darecchio S, Lui S, Basirico ML, Cocconi S, Cagossi K et al (2019) Scalp cooling in daily clinical practice for breast cancer patients undergoing curative chemotherapy: a multicenter interventional study. Asia Pac J Oncol Nurs 6(3):277–282
van den Hurk CJ, Peerbooms M, van de Poll-Franse LV, Nortier JW, Coebergh JW, Breed WP (2012) Scalp cooling for hair preservation and associated characteristics in 1411 chemotherapy patients: results of the Dutch Scalp Cooling Registry. Acta Oncol 51(4):497–504
Komen MM, Breed WP, Smorenburg CH, van der Ploeg T, Goey SH, van der Hoeven JJ, Nortier JW, van den Hurk CJ (2016) Results of 20- versus 45-min post-infusion scalp cooling time in the prevention of docetaxel-induced alopecia. Support Care Cancer 24(6):2735–2741
van den Hurk CJ, Breed WP, Nortier JW (2012) Short post-infusion scalp cooling time in the prevention of docetaxel-induced alopecia. Support Care Cancer 20(12):3255–3260
Bitto FF, Konig A, Phan-Brehm T, Vallbracht T, Koch JG, Schinkothe T, Wolfgarten M, Mahner S, Harbeck N, Wurstlein R (2020) EVA-scalp: evaluation of patient satisfaction with a scalp cooling device to prevent chemotherapy-induced alopecia in breast cancer patients. Breast Care 15(2):171–177
Komen MM, Smorenburg CH, van den Hurk CJ, Nortier JW (2013) Factors influencing the effectiveness of scalp cooling in the prevention of chemotherapy-induced alopecia. Oncologist 18(7):885–891
Komen MMC, van den Hurk CJG, Nortier JWR, van der Ploeg T, Nieboer P, van der Hoeven JJM, Smorenburg CH (2019) Prolonging the duration of post-infusion scalp cooling in the prevention of anthracycline-induced alopecia: a randomised trial in patients with breast cancer treated with adjuvant chemotherapy. Support Care Cancer 27(5):1919–1925
van den Hurk C, Keizer-Heldens P, Raats I, Hoeijmakers K, Mols F (2019) Improving information provision on chemotherapy-induced alopecia and scalp cooling: a comprehensive approach including a website and web-based decision tool. Asia Pac J Oncol Nurs 6(4):336–342
Tallon B, Blanchard E, Goldberg LJ (2010) Permanent chemotherapy-induced alopecia: case report and review of the literature. J Am Acad Dermatol 63(2):333–336
Freites-Martinez A, Chan D, Sibaud V, Shapiro J, Fabbrocini G, Tosti A, Cho J, Goldfarb S, Modi S, Gajria D et al (2019) Assessment of quality of life and treatment outcomes of patients with persistent postchemotherapy alopecia. JAMA Dermatol 155(6):724–728
Kang D, Kim IR, Choi EK, Im YH, Park YH, Ahn JS, Lee JE, Nam SJ, Lee HK, Park JH et al (2019) Permanent chemotherapy-induced alopecia in patients with breast cancer: a 3-year prospective cohort study. Oncologist 24(3):414–420
Martin M, de la Torre-Montero JC, Lopez-Tarruella S, Pinilla K, Casado A, Fernandez S, Jerez Y, Puente J, Palomero I, Gonzalez Del Val R et al (2018) Persistent major alopecia following adjuvant docetaxel for breast cancer: incidence, characteristics, and prevention with scalp cooling. Breast Cancer Res Treat 171(3):627–634
Acknowledgements
We thank all patients for participating in the study.
Funding
This study was funded by the Estée Lauder University Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
The study was approved by the Ethics Committee of UZ/KU Leuven.
Informed consent
Written informed consent to participate was obtained from all patients participating in the evaluation of patient-reported data. Written informed consent for publication of their details was obtained from all patients participating in the evaluation of patient-reported data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Coolbrandt, A., T’Jonck, A., Blauwens, K. et al. Scalp cooling in breast cancer patients treated with docetaxel–cyclophosphamide: patient- and nurse-reported results. Breast Cancer Res Treat 186, 715–722 (2021). https://doi.org/10.1007/s10549-020-06063-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-06063-w