Abstract
Introduction
Neoadjuvant chemotherapy (NAC) is a well-established therapeutic option for patients with locally advanced disease often allowing downstaging and facilitation of breast conserving therapy. With evolution of better targeted treatment regimens and awareness of improved outcomes for significant responders, use of NAC has expanded particularly for triple negative and HER2-positive (HER2+) breast cancer. In this study, we explore utility of neoadjuvant chemotherapy for hormone receptor-positive HER2-negative (HR+ HER2−) patients.
Methods
Patients with HR+ HER2− breast cancer treated with chemotherapy before or after surgery were identified from 2010 to 2015 in the NCDB. Multivariable regression models adjusted for covariates were used to determine associations within these groups.
Results
Among 134,574 patients (clinical stage 2A, 64%; 2B, 21%; 3, 15%), 105,324 (78%) had adjuvant chemotherapy (AC) and 29,250 (22%) received NAC. Use of NAC increased over time (2010–2015; 13.2–19.4% and PR = 1.34 for 2015; p < 0.0001). Patients were more likely to receive NAC with cT3, cT4, and cN+ disease. Patients less likely to receive NAC were age ≥ 50, lobular carcinoma, increased Charlson-Deyo score, and government insurance. Complete response (pCR) was noted in 8.3% of NAC patients. Axillary downstaging occurred in 21% of patients, and predictors included age < 50 years, black race, poorly differentiated grade, invasive ductal histology, and either ER or PR negativity.
Conclusions
NAC use among HR+ HER2− breast cancer patients has expanded over time and offers downstaging of disease for some patients, with pCR seen in only a small subset, but downstaging of the axilla in 21%. Further analysis is warranted to determine the subgroup of patients with HR+ HER2− disease who benefit from this approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Historically, neoadjuvant chemotherapy (NAC) has been used to downstage locally advanced breast cancer, which by definition involves the skin, chest wall, or multiple axillary lymph nodes [1]. Subsequent studies have found similar outcomes in recurrence and survival for treatment of operable breast cancer in the neoadjuvant versus the adjuvant setting [2, 3]. These findings paved the way for NAC use in multiple settings: downstaging larger tumors and facilitating breast conservation therapy (BCT) [2, 4], downstaging the axilla to avoid axillary lymph node dissection (ALND) [5,6,7], allowing for response monitoring and potential switch to a non-cross-resistant regimen in non-responders [8,9,10], and expediting approval of new drugs using pathologic complete response (pCR) as an endpoint [11, 12].
Hormone receptor-positive breast cancer is the most common molecular subtype, comprising 70–80% of all breast cancer diagnoses [13]. It is recognized that HR+ HER2− tumors are less likely to achieve pCR with NAC than other biologic subtypes [4, 6]. Triple negative breast cancer (TNBC) and HER2+ breast cancer have higher rates of pCR, on the order of 30–60% with chemotherapy and HER2 receptor blocking agents, versus less than 20% of those with HR-positive disease [14]. With evolution of better targeted treatment regimens, particularly for TNBC and HER2+ cancers, NAC use has expanded in these subtypes [15].
For patients with early-stage HR+ HER2− tumors, primary surgery rather than NAC is more common given that those patients are likely to have overall better prognosis than other phenotypes, significant response to endocrine therapy, and typically poor response to chemotherapy. In treating hormone receptor-positive breast cancer NAC is being offered increasingly with the hopes of improving the rates of tumor downsizing and less extensive surgery. However, there is a paucity of large population data in the national setting as to demographics, practice patterns, and outcomes for this specific population. We aim to clarify the demographic and clinicopathologic factors that predict choice of NAC versus adjuvant chemotherapy (AC) for treatment in HR+ HER2− breast cancer.
Methods
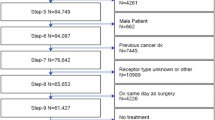
We identified the cohort of women from NCDB diagnosed with HR+ HER2− breast cancer from 2010 to 2015 based on corresponding variables in the Collaborative Stage Coding Manual. The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. It is a clinical oncology database sourced from hospital registry data that are collected in more than 1500 Commission on Cancer-accredited facilities. NCDB data are used to analyze and track patients with malignant neoplastic diseases, their treatments, and outcomes, and represent more than 70% of newly diagnosed cancer cases nationwide and more than 34 million historical records. 2010 was chosen as the start date for our study as it was the first year that the NCDB collected HER2 status data. We used the surgical procedure of the primary site code to stratify patients who did and did not receive surgical treatment. Only patients with both chemotherapy and surgery completed at the primary site within 8 months of diagnosis were included in the analysis. Patients who received chemotherapy before surgery were identified as NAC patients and those who had chemotherapy within 6 months of surgery were identified as AC patients. We restricted our cohort to only include clinical stage 2A, 2B and 3 patients and excluded missing pathologic T or N stage, path Tx or Nx and DCIS.
Potentially relevant demographic variables included age, race, insurance type, median household income, Charlson-Deyo comorbidity score, and year of diagnosis. Tumor-level variables included size, histology, grade and regional nodal involvement, and facility-level variables included distance from medical facility, facility type, and facility location. Clinical TNM was used for staging prior to treatment and in more modern cases positive node status may have been verified by axillary lymph node core needle biopsy. Pathologic TNM was used for determination of pCR.
Multiple imputation with chained equations, via IVEware software [16], was used to handle missing data in prognostic covariates. The imputation process loops through every variable containing missing values, where missing values were imputed using regression models conditional on all other variables. Ten imputed datasets were generated through ten repetitions. This method is superior to alternatives (complete case or missing data indicator methods) as far as analytic bias is concerned, under the assumption that data are missing at random [17]. The subsequent analyses were performed on each of the ten imputed datasets and resulting effect estimates and their corresponding 95% confidence intervals were appropriately combined using the MIANALYZE procedure in SAS.
Baseline prognostic variables were summarized within each treatment group as N (%).Separate multivariable Poisson regression models with a robust error variance were used to estimate adjusted prevalence ratios (PRs) and 95% confidence intervals (CIs) of factors related to utilization of NAC, in-breast pCR, and axillary downstaging within the NAC cohort, adjusting for variables outlined in the prognostic variables section. We chose PR over odds ratio as the latter tends to overestimate the strength of the association [18, 19].
Results
The baseline characteristics of NAC and AC cohorts are summarized in Table 1. Of the 134,574 eligible patients that received chemotherapy during the study period, 105,324 (78.3%) received AC and 29,339 (21.7%) received NAC. The use of NAC steadily increased over the study period from 19.7% in 2010 to 24.2% in 2015, an absolute increase of 4.5% (PR = 1.34, 95% CI 1.30, 1.38, for 2015 compared to 2010).
NAC use increased with clinical T stage three or four and with node positivity. Increasing clinical stage likewise had a positive association with NAC use. Other baseline tumor-related characteristics associated with increased NAC use include poorly differentiated tumors, either estrogen receptor (ER) or progesterone receptor (PR) negativity and invasive ductal histology. Baseline patient-related characteristics associated with increased NAC use include Black or Hispanic race, and Charlson score of 0, while age ≥ 50 years, median household income of < $38,000, and government insurance (i.e., Medicare and Medicaid) indicated decreased NAC use. Facility-related characteristics associated with increased NAC use include treatment at an academic or integrated facility and treatment at a facility in the central or western US.
Factors predicting pCR and axillary downstaging for NAC patients
Of the 29,250 patients that received NAC, 2401 (8.3%) patients achieved a pCR (Table 2). We defined pCR as ypT0 N0. pCR rates likewise increased over the study period from 6.3% in 2010 to 9.7% in 2015, an absolute increase of 3.4% (PR = 1.34, 95% CI 1.17, 1.53, for 2015 compared to 2010). pCR decreased with clinical T stage but increased with clinical N stage. Patients were more likely to achieve pCR if they were Black or Hispanic, resided in an urban location, had a poorly differentiated tumor, had invasive ductal histology, and had ER or PR negativity. Patients ≥ 50 years old or of Asian race were less likely to have a pCR.
Clinically node-positive patients receiving NAC converted to pathologically node negative 21% of the time (Table 3). Predictors of axillary downstaging include age < 50 years, black race, poorly differentiated grade, invasive ductal histology, and either ER or PR negativity (Table 4).
Overall 39.3% of patients underwent BCT and 60.7% had mastectomy. Of patients that had AC 29.4% had BCT, while patients in the NAC group had a 38.6% BCT rate. 60.4% of AC patients and 45.3% of NAC patients underwent ALND. Patients who underwent mastectomy or ALND were found to be less likely to achieve in-breast or nodal pCR.
Discussion
Breast cancer molecular subtypes have played a vital role in our modern appreciation of the disease and our approach to its treatment. Our understanding of these subtypes has promoted a shift toward personalized breast cancer care as opposed to historic methodology. Knowledge of patient molecular phenotype and subtype improves selection of treatment and prognostication of disease-specific outcome.
Our study shows an increasing trend in the use of NAC for patients with HR+ HER2− breast cancer, despite reported pCR rates of 10–20% [4, 6, 12, 20], and only 8.3% in our study. While pCR is predictive of a favorable prognosis, this relationship is stronger in more aggressive subtypes [12, 21, 20, 22, 23]. This can be largely attributed to the fact that the HR-positive subtype has a favorable prognosis regardless of pCR [24]. With pCR rates of 30–40% in TNBC and over 50% in HER2+ breast cancer, it is not surprising that enthusiasm for NAC in these excellent responders has increased over time [15, 25]. The positive trend for NAC use in HR+ HER2− breast cancer is less an attempt to achieve a pCR but rather the intent to downstage the tumor to avoid mastectomy and/or downstage the axilla to avoid ALND.
The ACOSOG Z1071 trial evaluated the impact of tumor biology on the rate of BCT following NAC offering some comparison [4]. The study included 694 patients of all breast cancer subtypes with clinically node-positive disease receiving NAC followed by surgery. The pCR rate in HR+ HER2− patients was 11.4%, compared to 38% for TNBC and 45% for HER2+ cancer. However, a clinical response (complete or partial) was seen in 80.5% of HR+ HER2− patients and a pathologic response was seen in 71.8%. Only 9.5% of these patients showed disease progression while on neoadjuvant therapy. The HR+ HER2− patients were also more likely to undergo mastectomy compared to their TNBC and HER2+ counterparts, at a rate of 65.5%. The retrospective analysis of this prospective study did not allow for discerning which patients were mastectomy or BCT candidates before NAC receipt, and therefore, it is difficult to draw conclusions from this finding. This study was also unable to address which patients were BCT candidates after NAC but elected mastectomy. In fact, the residual tumor size in patients that did not achieve a pCR was similar across all subtypes. One hypothesis for the higher mastectomy rate in HR+ HER2− patients was the higher proportion of invasive lobular carcinoma seen in this subtype, which required more second procedures than patients with invasive ductal carcinoma.
A prospective, single-center study determined the frequency of avoiding ALND in clinically node-positive breast cancer patients that received NAC [6]. They included 155 patients spanning all tumor subtypes, with HR+ HER2− (56%) and invasive ductal histology (95%). The overall rate of nodal pCR was 49% but varied significantly by HR and HER2− receptor status. The rate of nodal pCR in HR+ HER2− cancer was 21%, exactly as seen in our study and in ACOSOG Z1071 [4]. While other subtypes appreciated higher rates of nodal pCR at 47%, 97%, and 70% for TNBC, HR-negative HER2+, and triple positive cancer, respectively, a nodal pCR seen in about one out of five patients with HR+ HER2− subtype is substantial.
Given the relatively lower rates of pCR and axillary downstaging in HR+ HER2− breast cancer treated with NAC, we continue to seek better therapeutic options for these patients. Neoadjuvant endocrine therapy (NET) offers a less toxic alternative as a potential management strategy, especially for postmenopausal women [26]. The ACOSOG Z1031 trial studied the effect of preoperative aromatase inhibitors (AI) on promoting BCT for 374 postmenopausal women with HR+ HER2− breast cancer [27]. Unlike Z1071, this trial designated patients’ pretreatment by their candidacy for BCT and compared it to the most extensive surgery actually performed. 50.9% of mastectomy-only candidates before treatment underwent BCT as their most extensive surgery, as well as 83.1% of marginal for BCT candidates. Three out of four inoperable candidates likewise were managed with BCT. This suggests marked improvements in surgical outcomes with NET with an overall BCT rate of 68%. There is also the potential to improve response rate with newer approaches such as selecting a subset of patients for NET using genomic profiling. Likewise, the ALTERNATE trial is a phase III randomized controlled trial that is seeking to obtain pCR and recurrence-free survival data in postmenopausal women on neoadjuvant AI [28].
Interestingly, our study found a higher rate of in-breast and axillary pCR for black patients compared to white patients. Several studies addressing the impact of race following NAC found no difference in pCR overall or by subtype [29,30,31,32,33]. They did, however, find that black patients receiving NAC consistently had worse outcomes even when matched for BMI, subtype, and stage.
While use of retrospective data from NCDB has limitations, this cohort included just under 135,000 patients which is the main strength of our study. Lack of data regarding compliance with treatment or duration of NAC and degree of estrogen/progesterone receptor expression are limitations. In addition, HER2 status was not collected by the NCDB before 2010; therefore, some HER2-positive patients are included.
NAC use for HR+ HER2− breast cancer has increased over time and, in spite of lower pCR rates compared to other phenotypes, offers a significant clinical benefit for many patients. Our study found that NAC should be strongly considered in patients with locally advanced HR+ HER2− disease who desire BCT or who are clinically node positive, especially if they are young or have poorly differentiated invasive ductal cancer with either ER or PR negativity. We expect improved outcomes going forward as better patient selection is guided by increasing use of pretreatment genomic assays. Black patients showed an improved rate of pCR compared to white patients despite prior studies finding that race did not influence pCR rate. More studies are needed to explore these findings and better predict patients who would be excellent responders.
References
Perloff M, Lesnick GJ (1982) Chemotherapy before and after mastectomy in stage III breast cancer. Arch Surg 117:879
Fisher B, Brown A, Mamounas E et al (1997) Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15:2483
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26:778
Boughey JC, McCall LM, Ballman KV et al (2014) Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (alliance) prospective multicenter clinical trial. Ann Surg 260(4):608–616
Donker M, Straver ME, Wesseling J et al (2015) Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg 261:378–382
Mamtani A, Barrio AV, King TA et al (2016) How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastasis? Results of a prospective study. Ann Surg Oncol 23:3467–3474
El Hage CH, Headon H, El Tokhy O et al (2016) Is sentinel lymph node biopsy a viable alternative to complete axillary dissection following neoadjuvant chemotherapy in women with node-positive breast cancer at diagnosis? An updated meta-analysis involving 3398 patients. Am J Surg 212:969
von Minckwitz G, Kummel S, Vogel P et al (2008) Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized GerparTrio trial. J Natl Cancer Inst 100:542–551
von Minckwitz G, Blohmer JU, Costa SD et al (2013) Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol 31:3623–3630
Rigter LS, Loo CE, Linn SC et al (2013) Neoadjuvant chemotherapy adaptation and serial MRI response monitoring in ER-positive HER2-negative breast cancer. Br J Cancer 109:2965–2972
Prowell TM, Pazdur R (2012) Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med 366:2438–2441
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172
Anderson E (2002) The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res 4:197–201
Schott AF, Hayes DF (2012) Defining the benefits of neoadjuvant chemotherapy for breast cancer. J Clin Oncol 30:1747
Murphy BL, Day CN, Hoskin TL et al (2018) Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: triple-negative and HER2+ subtypes. Ann Surg Oncol 25(8):2241–2248
White IR, Royston P, Wood AM (2011) Multiple imputation using chained equations: issues and guidance for practice. Stat Med 30:377–399
van der Heijden GJ, Donders AR, Stijnen T, Moons KG (2006) Imputation of missing values is superior to complete case analysis and the missing indicator method in multivariable diagnostic research: a clinical example. J Clin Epidemiol 59:1102–1109
Guangyong Z (2004) A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159(7):702–706
Tamhane AR, Westfall AO, Burkholder GA, Cutter GR (2016) Prevalence odds ratio versus prevalence ratio: choice comes with consequences. Stat Med 35(30):5730–5735
Esserman LJ, Berry DA, Cheang MC et al (2012) Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res 132:1049–1062
Kuerer HM, Newman LA, Buzdar AU et al (1998) Resideual metastatic axillary lymph nodes following neoadjuvant chemotherapy predict disease-free survival in patients with locally advanced breast cancer. Am J Surg 176:502–509
Spring LM, Fell G, Arfe A, et al (2018) Pathological complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival, stratified by breast cancer subtypes and adjuvant chemotherapy usage. San Antonio Breast Cancer Symposium. Presented December 5, 2018.
von Minckwitz G, Untch M, Blohmer JU et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804
Fisher B, Jeong JH, Bryant J et al (2004) Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. Lancet 364:858–868
Puig C, Hoskin TL, Day CN et al (2017) National trends in the use of neoadjuvant chemotherapy for hormone receptor-negative breast cancer: a national cancer data base study. Ann Surg Oncol 24:1242–1250
Chiba A, Hoskin TL, Heins CN et al (2017) Trends in neoadjuvant endocrine therapy use and impact on rates of breast conservation in hormone receptor-positive breast cancer: a national cancer data base study. Ann Surg Oncol 24:418–424
Ellis MJ, Suman VJ, Hoog J et al (2011) Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype-ACOSOG Z1031. J Clin Oncol 29(17):2342–2349
Suman VJ, Ellis EJ, Ma CX (2015) The alternate trial: assessing a biomarker driven strategy for the treatment of post-menopausal women with ER+/Her2- invasive breast cancer. Chin Clin Oncol 4(3):34
Chavez-MacGregor M, Litton J, Chen H et al (2010) Pathologic complete response in breast cancer patients receiving anthracycline- and taxane-based neoadjuvant chemotherapy. Cancer 116:4168–4177
Warner ET, Ballman KV, Strand C et al (2016) Impact of race, ethnicity, and BMI on achievement of pathologic complete response following neoadjuvant chemotherapy for breast cancer: a pooled analysis of four prospective Alliance clinical trials (A151426). Breast Cancer Res Treat 159:109–118
Killelea BK, Yang VQ, Wang SY et al (2015) Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: results from the National Cancer Data Base. J Clin Oncol 33:4267–4275
Tichy JR, Deal AM, Anders CK et al (2015) Race, response to chemotherapy, and outcome within clinical breast cancer subtypes. Breast Cancer Res Treat 150:667–674
Pastoriza JM, Karagiannis GS, Lin J et al (2018) Black race and distant recurrence after neoadjuvant or adjuvant chemotherapy in breast cancer. Clin Exp Metas 25:613–623
Acknowledgements
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All author declares that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeidman, M., Alberty-Oller, J.J., Ru, M. et al. Use of neoadjuvant versus adjuvant chemotherapy for hormone receptor-positive breast cancer: a National Cancer Database (NCDB) study. Breast Cancer Res Treat 184, 203–212 (2020). https://doi.org/10.1007/s10549-020-05809-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05809-w