Abstract
Purpose
Breast cancer brain metastases (BCBM) are becoming an increasingly common diagnosis due to improved systemic control and more routine surveillance imaging. Treatment continues to require a multidisciplinary approach managing systemic and intracranial disease burden. Although, improvements have been made in the diagnosis and management of BCBM, brain metastasis patients continue to pose a challenge for practitioners.
Methods
In this review, a group of medical oncologists, radiation oncologists, radiologists, breast surgeons, and neurosurgeons specializing in the treatment of breast cancer reviewed the available published literature and compiled a comprehensive review on the current state of BCBM.
Results
We discuss the pathogenesis, epidemiology, diagnosis, treatment options (including systemic, surgical, and radiotherapy treatment modalities), and treatment response evaluation for BCBM. Furthermore, we discuss the ongoing prospective trials enrolling BCBM patients and their biologic rationale.
Conclusions
BCBM management is an increasing clinical concern. Multidisciplinary management combining the strengths of surgical, systemic, and radiation treatment modalities with prospective trials incorporating knowledge from the basic and translational sciences will ultimately lead to improved clinical outcomes for BCBM patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of brain metastases from breast cancer has become an increasingly common occurrence due to improvements in systemic therapy and advances in imaging techniques [93]. Breast cancer accounts for roughly 17% of brain metastases, the second most common cause after lung cancer [93, 146]. Approximately 15–30% of breast cancer patients will develop brain metastases during the course of their disease [113, 130]. Although a multitude of systemic treatment options exist for extracranial breast metastases, brain metastases continue to pose management challenges due to the myriad of treatment options available, which require a patient-specific multidisciplinary approach involving medical oncology, radiation oncology, and neurosurgery for optimal management. In this review, we discuss the current approach to diagnosis, multidisciplinary management, and future directions for treatment.
Prognosis
Efforts to ascertain which patients are at an increased risk for central nervous system (CNS) involvement have identified human epidermal growth factor receptor 2 (HER2) positivity, triple-negative (TN) tumors, young age, lymph node involvement, high grade, and increased tumor size as significant risk factors [41, 51, 52]. There is significant heterogeneity within the clinical outcomes for patients with breast cancer brain metastasis (BCBM), as the median overall survival (OS) ranges from 3 to 26 months [127]. There are multiple prognostic models for patients who develop brain metastasis, including the Recursive Partitioning Analysis (RPA) [42] and the Graded Prognostic Assessment (GPA) [122], which both identified age, performance status, and extracranial disease as significant prognostic factors. Studies have established that receptor status can stratify clinical outcomes, as TN tumors have relatively poor OS, with a median of 3–6 months, while HER2-positive tumors had significantly improved outcomes due to targeted therapy, with a median OS of 11–18 months [28, 65, 85, 94, 117, 145]. The most recent breast cancer-specific prognostic model, the Modified Breast GPA, confirmed that tumor receptor status, along with age, performance status, and number of brain metastases, predicted OS [127].
Diagnosis and screening
Current NCCN guidelines recommend brain MRI screening for recurrent or stage IV breast cancer patients only if symptoms are present [87]. This differs from recommendations for SCLC, stage ≥ II non-small-cell lung cancer (NSCLC), as well as stage IIIC–IV melanoma [88,89,90]. As the CNS has been an increasing site of failure of breast cancer due to improved systemic control, particularly in HER2 + and TN tumors, there may be a role for routine brain MRI screening in advanced and recurrent breast cancer. A study by Martin et al. reviewed the experience of breast and NSCLC brain metastases management and found that breast cancer patients at initial brain metastases diagnosis were noted to have larger and more numerous brain metastases compared to NSCLC brain metastases [77]. In addition, breast cancer patients were more likely to have symptoms at diagnosis including seizures, leptomeningeal disease, and brainstem involvement. Due to more numerous, larger, and symptomatic brain metastases, breast cancer patients compared to NSCLC patients were more likely to receive whole brain compared to stereotactic radiation. Given these findings, the role of screening brain MRIs, particularly in higher risk populations, should be brought into question. Current prospective trials are studying the role of screening MRIs in breast cancer of various subtypes (clinicaltrials.gov identifier: NCT04030507) and in HER2 + and triple-negative subtypes (NCT03881605).
Breast cancer and the brain microenvironment
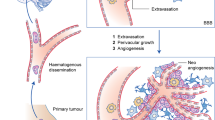
The importance of the dynamic interactions within the tumor microenvironment has been established for multiple cancers, and improved understanding of these complex interactions has the potential to aid the search for effective BCBM treatment [143]. However, relatively little is known about the tumor microenvironment within the brain, a setting with obstacles unique to any other metastatic site. Perhaps the most distinctive feature of the CNS is the blood–brain barrier (BBB), a selective diffusion barrier of the cerebral microvascular endothelium that poses a significant obstacle to CNS penetration for metastases [110]. However, tumor cell extravasation in the brain is possible through a variety of mechanisms [14, 61, 124]. Key mediators of CNS penetration for BCBM include the cyclooxygenase COX2, the epidermal growth factor (EGFR) ligand HBEGF, the α2,6-sialyltransferase ST6GALNAC5, and specific matrix metalloproteinases, among others [14, 124]. The ability for tumor cells to cross the BBB, along with the significant delay between the first appearance of circulating tumor cells and the detection of brain metastasis, suggests that tumor colonization within the brain is a crucial step of metastasis formation [134].
Successful colonization depends upon the interactions of tumor cells with unique supportive glial cells of the CNS, microglia and astrocytes [39, 107, 134]. Xing et al. demonstrated that brain metastases expressing a high level of c-Met, a receptor tyrosine kinase, induce a feed-forward cycle of cytokine release with tumor-associated astrocytes that created a favorable microenvironment for tumor cells [147]. Another study found that upregulated c-Met signaling and expression was associated with radioresistance in BCBM cells [149]. Targeting c-Met alone reduced tumor growth, and in combination with radiotherapy, prolonged survival in a murine model [147, 149]. Sikisoon et al. investigated the role of truncated glioma-associated oncogene homolog 1 (TGLI1), a transcription factor associated with angiogenesis, migration, and invasion, in BCBM [118]. Two murine models showed that increased activation of TGLI1 was associated with HER2 + and TNBC, relative radioresistance, and a shortened time to development of BCBM. Astrocyte activation played a significant role in the role of TGLI1 in BCBM formation.
Studies have also demonstrated important interactions between brain metastases and neurons [139]. Neman et al. found that BCBM cells displayed high expression of gamma-Aminobutyric acid (GABA) receptors, transporters, and transaminase, similar to neuronal cells [92]. This increased expression allowed metastases to catabolize GABA and form nicotinamide adenine dinucleotide (NADH), which increased cell proliferation. Zeng et al. demonstrated that triple-negative BCBMs hijack a neuronal signaling pathway by expressing N-methyl-d-aspartate receptors (NMDARs), which are activated by high amounts of glutamate within neuronal synapses [150]. This NMDAR signaling was shown to promote brain colonization and metastasis growth.
Treatment response evaluation
The assessment of treatment response for patients with brain metastasis after radiotherapy may be challenging. Post-radiation effects, such as pseudoprogression, a transient disruption to myelin synthesis and radiation necrosis, necrotic lesions that cause mass effect and neurologic dysfunction, can mimic tumor recurrence upon post-treatment magnetic resonance imaging (MRI) [100]. There has been recent interest in functional imaging methods, such as diffusion weight imaging (DWI), dynamic susceptibility-weighted contrast-enhanced (DSC) MR imaging, and dynamic contrast-enhanced (DCE) MR imaging, to better differentiate between tumor recurrence and radiation treatment effects [141]. Quantitative analysis of post-treatment positron emission tomography (PET) and MRI scans have shown potential to improve the diagnostic accuracy of brain metastasis recurrence as well [71, 72]. However, these methods lack prospective validation. Post-radiation response evaluation remains a challenge, and accurate differentiation between radiation necrosis, pseudoprogression, and true recurrence relies upon clinical assessment and repeated imaging exams [100, 141].
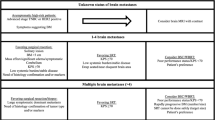
A critical component in the search for new treatments for BCBM patients is the establishment of a consistent treatment response evaluation criteria. The Response Assessment in Neuro-Oncology (RANO) group developed response criteria specifically for brain metastases (RANO-BM), which considers target and non-target lesion response, steroid use, and neurologic symptoms (Table 1) [69]. However, there are important limitations of the RANO-BM in regards to the assessment of progressive disease in patients receiving immunotherapy. Studies have demonstrated that early radiologic findings of disease progression, including increased size of the target lesion or the development of new lesion, do not always accurately predict therapeutic benefit [95]. Therefore, the immunotherapy Response Assessment in Neuro-Oncology (iRANO) criteria were developed to increase the accuracy in identifying true disease progression [96]. For patients who demonstrate radiologic signs of progressive disease without clinical decline and within 6 months of initiating immunotherapy, confirmatory imaging is required 3 months after initial radiologic evidence of progression. The iRANO criteria does require prospective validation prior to widescale adoption.
Local management of BCBM
The treatment options for patients with BCBM have traditionally relied on local approaches including surgery, whole brain radiation therapy (WBRT), and stereotactic radiosurgery (SRS). Surgery can improve survival but is typically reserved for those patients with symptoms, optimal performance status and limited brain metastatic disease [102]. Whether or not they undergo surgical resection, BCBM patients receive radiotherapy. Patients with acceptable performance status and more localized disease undergo SRS, while patients with extensive intracranial disease or poor performance status typically receive WBRT. Traditionally, the role of systemic therapy for BCBM patients has been limited due to the challenges posed by the BBB [31, 70]. However, the role of systemic therapy in management of BCBM patients has rapidly evolved following recent successes in studies managing HER2 + BCBM patients [9, 63, 81].
Surgery
Three randomized clinical trials investigated the additional benefit gained by surgical resection prior to WBRT for patients with a single brain metastasis [80, 102, 140]. One trial conducted by Mintz et al. failed to demonstrate a significant difference in survival or Karnofsky performance status (KPS) between those who received WBRT alone compared to those who received the combination treatment [80]. However, the other two trials demonstrated that patients who received surgical resection had improved OS, as well as more rapid improvement and longer duration of functional independence improvement [102, 140]. Therefore, patients with acceptable performance status and limited extracranial disease are advised to undergo resection followed by radiotherapy, and there’s evidence that those patients with symptomatic large lesions (≥ 3 cm in diameter) will benefit most from resection [57, 120].
Several studies have called into question whether prior surgical resection followed by stereotactic radiation may raise the risk of nodular leptomeningeal disease. Nodular or pachymeningeal leptomeningeal disease differs from classical leptomeningeal disease, which appears as tumor masses in the extra-axial spaces near the site of surgical resection. This type of leptomeningeal spread is thought to arise directly from microscopic tumor spillage from surgery or in the post-operative period. A change in the practice from delivering WBRT rather than focal stereotactic radiation may make this pattern of spread more common [105]. In a study by Cagney et al., the incidence of nodular leptomeningeal disease was noted in 36 (8.4%) of 428 operations, with a higher incidence noted with resection of previously irradiated vs unirradiated metastases (p = .008) [21]. Interestingly, pre-operative SRS has been shown to have lower rates of LMD formation compared to post-SRS (2 years: 16.6% vs 3.2%, p = .010) [103], though further prospective studies assessing this approach are warranted.
Radiotherapy
Historically, WBRT has been the standard treatment for patients with brain metastasis. Patchel et al. demonstrated the addition of WBRT to surgery improved rates of intracranial recurrence, and while OS was not significantly different between the two arms, WBRT decreased the rates of neurologic death [101]. The role of WBRT has decreased in recent years due to treatment-related toxicities, including somnolence and impairments to short-term memory, and therefore, WBRT is more often utilized for patients with numerous brain metastases and poor performance status. However, there have been attempts to reduce the rates of neurocognitive toxicity. A placebo-controlled trial found that patients treated with WBRT who received memantine, an N-methyl-d-aspartate glutamate receptor blocker typically utilized in dementia, had delayed time to cognitive decline and reduced rates of impairments to memory, executive function, and processing speed [18]. RTOG 0933, a single-arm phase II study, found that intensity-modulated radiotherapy (IMRT) techniques to avoid the hippocampus significantly decreased rates of cognitive decline when compared to historical controls of standard WBRT [47]. Preliminary results from the phase III NRG Oncology CC001 trial reveal improved neurocognitive function in the arm randomized to hippocampal avoidance WBRT compared to standard WBRT [46].
The role of SRS for patients with brain metastasis has been expanding due to its highly conformal nature that spares a significant volume of healthy brain tissue combined with high local control rates. A phase III trial found that when compared to observation, post-operative SRS improved local control for patients with 1–3 brain metastases, similar to WBRT [76]. When compared to WBRT, two additional trials demonstrated no significant differences in OS after SRS [16, 59]. While WBRT was associated with improved intracranial progression free survival (PFS), SRS was associated with a lower risk of cognitive decline. SRS may be an option for patients with up to 10 brain metastases, as a prospective observational study conducted across 23 Japanese institutions in over 1100 patients with 1–10 brain metastases treated with SRS demonstrated that OS did not differ between patients with 2 to 4 metastases and those with 5–10 (median OS 10.8 months in both groups) [148]. There were similar rates of toxicity, local control, neurologic death, leptomeningeal dissemination, and use of salvage radiotherapy between the two groups.
There have been multiple trials investigating the utility of treating patients with both WBRT and SRS. In a phase III trial, Andrews et al. demonstrated the addition of SRS to WBRT significantly improved OS for patients with 1–3 brain metastases and good prognosis (GPA 3.5–4.0) [5, 123]. Multiple trials have established that for patients with 4 or fewer brain metastases, the addition of WBRT to SRS improves both local failure and distant brain failure rates [6, 17, 23, 62]. However, when compared to SRS alone, the addition of WBRT increases the risk of neurocognitive toxicity without conferring a benefit to OS [17]. Interestingly, a combined analysis of three prospective trials found that for patients 50 years or younger, the addition of WBRT to SRS did not impact distant brain relapse rates, and patients who received SRS alone had improved OS [114]. Due to these results, the most recent consensus guidelines recommend against adjuvant WBRT following complete resection or SRS in favor of close monitoring for patients with a limited number of brain metastases [120].
Systemic therapy
Drug conjugates
Historically, the focus of BCBM treatment has been local treatment via surgery and/or radiation therapy due to the challenges posed by the BBB [31, 70]. After a metastasis forms, the BBB remnants, called the blood-tumor barrier (BTB), exhibit increased permeability [91]. While BTB permeability is increased with WBRT and surgery, systemic drug penetration is highly variable and rarely reaches cytotoxic concentrations [70, 81].
There have been multiple recent efforts to overcome the barriers posed by the BTB. ANG1005, a peptide-drug conjugate treatment including Angiopep-2 covalently linked to paclitaxel, utilized the low-density lipoprotein receptor-related protein 1 (LRP-1) to cross the BBB [136]. A phase 2 study of patients with metastatic breast cancer and recurrent brain metastasis found that ANG1005 demonstrated clinical activity, with 14% and 57% of patients with partial response and stable disease, respectively [64]. Patients with leptomeningeal carcinomatosis who received ANG1005 had a median OS of 8 months, longer than the historical median of 4 months following traditional treatment. An ongoing phase 3 clinical trial is further investigating the role of ANG1005 in treating patients with HER2-negative breast cancer and newly diagnosed leptomeningeal carcinomatosis and previously treated brain metastasis (NCT03613181).
Etirinotecan pegol (NKTR 102), a next generation topoisomerase-1 inhibitor-polymer conjugate, also enables BBB penetration and allows for the delivery of SN38, the active metabolite of irinotecan. The BEACON phase 3 trial randomized treatment with NKTR-102 compared physician’s choice treatment for women with advanced breast cancer previously treated with an anthracycline, a taxane, and capecitabine [104]. Unfortunately, NKTR-102 did not significantly improve OS. However, a subgroup analysis demonstrated NKTR-102 drastically improved OS for patients with BCBM within the trial, with a median OS of 10 months compared to 4.8 months [26]. An ongoing phase 3 trial, ATTAIN, is underway to further study the efficacy of NKTR-102 for patients with metastatic breast cancer with stable brain metastases. (NCT02915744).
HER2-positive BCBM
HER2 is a member of the EGFR family and a membrane tyrosine kinase. This oncogene is overexpressed in approximately 14% of breast cancers and is associated with higher risk of brain metastasis, as roughly 44% of resected brain metastases are HER2-positive [60, 117, 121]. While this remains an aggressive subset of breast cancer, HER2 targeted therapies have significantly improved rates of OS; however, anti-HER2 therapy has limited CNS penetration. HER2 + positive patients have among the highest rates of brain metastasis failure [7]. Studies show that the CSF concentration of trastuzumab is 1:420 without pretreatment and improves to 1:79 after being treated with radiation or surgery [35, 131]. To circumvent these low concentrations, in the setting of LMD, intrathecal delivery of trastuzumab has been utilized which has shown improvement in OS compared to historical controls in retrospective analyses and in a phase I/II prospective trial [12, 37, 64].
A phase III trial demonstrated that treatment with both trastuzumab and pertuzumab, two HER2-targeted monoclonal antibodies, combined with docetaxel improved both OS and PFS when compared with trastuzumab and docetaxel alone for patients with metastatic HER2-positive breast cancer [129]. While this trial excluded patients with brain metastasis, an exploratory analysis found the addition of pertuzumab delayed the development of CNS metastasis as the first site of progression [128]. A prospective observational study of the registHER, a database of over 1000 patients with HER2-positive breast cancer, found that trastuzumab and chemotherapy both significantly improved OS for patients with CNS metastasis [19]. This improvement in survival is likely due to improvement in extracranial disease control rather than a direct effect on brain metastases [99]. However, there has been recent interest in the utilization of radiotherapy or ultrasound techniques to increase the permeability of the BTB to trastuzumab that warrant continued investigation [98, 125].
There has been promising data for the treatment of metastatic HER2 + breast cancer patients with trastuzumab emtansine (T-DM1), an antibody–drug conjugate with the HER2-targeted antitumor properties of trastuzumab and the cytotoxic activity of DM1, a potent microtubule inhibitor. The EMILIA phase III trial randomized 991 patients with HER2-positive advanced breast cancer to either treatment with either T-DM1 or lapatinib and capecitabine [142]. Patients treated with T-DM1 had superior PFS (median 9.6 months vs 6.4 months, p < 0.001), OS (median 30.9 months vs 25.1 months, p < 0.001), and objective response rate (ORR) (43.6% vs 30.8%, p < 0.001). This trial excluded patients with CNS metastases that were symptomatic or recently treated. However, an exploratory analysis of the 95 patients who had CNS metastasis at baseline demonstrated that, while there were similar rates of CNS progression between the treatment arms, patients treated with T-DM1 had improved OS (median 26.8 months vs 12.9 months, p = 0.008), likely via superior extracranial disease control [63]. A recent analysis has questioned whether stereotactic radiation combined with T-DM1 may increase the risk of radionecrosis through T-DM1 targeting reactive astrocytes and increasing radiation-induced cytotoxicity and astrocytic swelling via upregulation of Aquaporin-4 (Aqp4) [126].
Small molecular tyrosine kinase inhibitors that inhibit the HER2 receptor have shown the potential to improve outcomes for HER2-positive breast cancer. One such tyrosine kinase inhibitor (TKI), lapatinib, targets both HER2 and EGFR. Three phase 3 clinical trials have demonstrated that lapatinib-based regimens, especially when combined with trastuzumab, can improve OS and PFS for HER2-positive locally advanced or metastatic breast cancer patients [11, 34, 43]. Lapatinib has the ability to cross the BTB, as concentrations are significantly higher within brain metastases compared to healthy brain tissue [132]. However, the penetration of the BTB is highly variable, and only about 17% of brain metastases demonstrated a lapatinib concentration that approached that of systemic metastases [132]. The LANDSCAPE trial investigated the treatment combination of lapatinib and capecitabine for 45 patients with HER2-positive BCBM not previously treated with WBRT, capecitabine, or lapatinib [9]. The trial demonstrated encouraging results, as 65.9% of patients had a partial CNS response defined as a ≥ 50% response to treatment with a median time to progression of 5.5 months. A phase I trial of HER2 + BCBM patients demonstrated that concurrent WBRT and lapatinib followed by adjuvant lapatinib and trastuzumab can achieve a CNS ORR of 79% and 6-month PFS of 46% [68].
Pooled analysis of two phase 1b studies of tucatinib, another small molecule TKI, in the treatment of metastatic HER2-positive breast cancer patients identified a subgroup of patients that achieved extended disease control, with a PFS of at least 16 months [48]. Within this subgroup, half of the patients had brain metastasis. Another pooled analysis of the two phase 1b studies demonstrated comparable PFS for patients with and without brain metastasis [83]. Further analysis investigated the outcomes for patients who developed isolated brain progression and received CNS-directed therapy [84]. Those patients that continued on the tucatinib-based regimen, compared to those who discontinued the study, had superior preserved performance status, reduced risk of neurologic adverse events, and a median of 8.3 months to any second event.
The phase 3 ExteNET trial established an additional year of adjuvant neratinib, an irreversible TKI with inhibition of HER1, HER2, and HER4, administered after chemotherapy and trastuzumab, improved invasive disease-free survival for patients with HER2-positive early stage breast cancer [78]. The NEfERT-T phase 3 trial randomized 479 women with previously untreated and/or recurrent or metastatic HER2-positive breast cancer to received neratinib or trastuzumab, each combined with paclitaxel [8]. While there was no significant difference in PFS, the neratinib group did have a lower incidence of CNS recurrence (RR 0.48, p = 0.002), as well as a longer time to CNS metastasis (HR 0.45, p = 0.004). A phase 2 trial of neratinib and capecitabine for patients with HER2 + BCBM demonstrated further evidence of CNS activity, as patients who were lapatinib-naïve achieved a CNS ORR of 49% and a median PFS of 5.5 months [40]. The phase 3 NALA trial, which randomized patients with stage 4 HER2-positive breast cancer to receive neratinib and capecitabine or lapatinib and capecitabine, demonstrated that patients treated in the neratinib arm had a significant improvement in PFS. Interestingly, patients treated with neratinib had decreased rates of CNS disease (22.8% vs 29.2%, p = 0.043) and delayed time to intervention for symptomatic CNS disease [27].
Afatinib, a novel TKI that targets both EGFR and HER4, has also been used to treat HER2-positive breast cancer. A phase II trial of patients with stage III or inflammatory disease found that for neoadjuvant treatment, afatinib compared favorably with trastuzumab monotherapy and lapatinib monotherapy [111]. However, further trials have demonstrated a suboptimal associated risk benefit ratio. The LUX-Breast 3 phase 2 trial randomized 121 patients with HER2-positive breast cancer and CNS recurrence or progression to treatment with afatinib, afatinib with vinorelbine, or the investigator’s choice of treatment [25]. The afatinib-containing regimens had higher rates of toxicity without improvement in patient benefit. Ongoing prospective clinical trials for HER2 + patients are detailed in Table 2.
Hormone-positive BCBM
Endocrine, or hormonal, therapy is an integral component in the treatment of HR + breast cancer at both the early and advanced stages of disease; however, the role of hormonal agents in the treatment of CNS metastases has not been well studied. While tamoxifen is highly lipophilic and able to cross the blood–brain barrier achieving therapeutic concentrations within the CSF, there is reasonable concern for hormonal resistance at time of progression [66]. Approximately 20% of HR + breast cancer patients have a de novo, or primary, resistance to hormone therapy [79], and nearly half of patients will ultimately acquire resistance with prolonged treatment [97]. Furthermore, there is concern whether hormonal therapy would be effective for CNS metastases as the discordance rates of hormone receptor status between the primary and metastatic intracranial tumors is approximately 40% [53, 56].
One therapeutic approach to combat hormonal resistance has been to target the downstream pathways by inhibiting CDK4 and CDK6. CDK4/6 inhibitors, such as abemaciclib, palbociclib, and ribociclib, have been shown to cause cell cycle arrest, decreased cell viability, and apoptosis [106]. Several large prospective trials have demonstrated improved ORR, PFS, and OS in both the endocrine-resistant (MONARCH-2 [119], PALOMA-3 [138]) and the first-line setting (MONARCH-3 [44], PALOMA-2 [38], and MONALEESA 7 [54]) for HR + advanced breast cancer patients.
The efficacy of CDK4/6 inhibitors on CNS metastases is currently unknown. Only the PALOMA-2, PALOMA-3, MONALEESA-3 and MONALEESA7 trials included patients with CNS disease. Preclinical and clinical data have demonstrated the ability of CDK4/6 inhibitors to cross the BBB with variable efficiency [30, 109, 137]. Given CDK4/6 inhibitor’s potential benefit and minimal side effects, prospective studies are ongoing to assess the efficacy of abemaciclib (NCT02308020) and palbociclib (NCT02774681) in the treatment of BCBM. Early results of NCT02308020, which were recently published in abstract form, found an intracranial clinical benefit rate of 25% with abemaciclib and a median PFS of 4.4 months in a heavily pretreated patient population [22].
However, preclinical evidence suggests that there may be a synergistic benefit combining CDK inhibitors with radiotherapy [49, 144]. As patients survive longer with improved systemic agents and more frequent imaging identifies a greater number of asymptomatic brain metastases, it is essential to ensure that the synergistic combination of CDK4/6 and RT does not translate to increased toxicities. Two recently published retrospective studies suggest that the early clinical experience in combining CDK4/6 inhibitors and radiotherapy is safe and tolerable. Chowdary et al. reported minimal grade 2 and no grade 3 toxicities in the 16 patients treated with conventionally fractionated radiotherapy, including three who received WBRT [24]. Figura et al. published their single institution experience treating 42 intracranial lesions with stereotactic radiotherapy within 15 patients concurrently taking CDK4/6 inhibitors reporting a 5% rate of radionecrosis, comparable to historical controls with OS that appeared improved [36].
As described above, endocrine resistant HR + breast cancer may proliferate by utilizing alternative cell signaling pathways. Recurrent HR + breast cancer is oftentimes associated with constitutionally active PI3K/AKT/mTOR pathways [1, 15], which may provide a novel therapeutic target in treating HR + BCBM. The use of PI3K inhibitor inhibitors, such as taselisib [20] or mTOR inhibitors, such as everolimus [10], have found to demonstrate a systemic response in prospective studies. Recent in vitro studies have demonstrated the ability for pan-Akt inhibitors to cross the BBB, decreasing tumor cell viability, and inducing apoptosis in BCBM [55]. Multiple prospective studies are currently evaluating their use of combining PI3K/AKT/mTOR and CDK4/6 inhibitors in HR + breast cancer to prevent treatment resistance (NCT03006172, NCT02684032, NCT02389842, NCT02732119, NCT02871791, NCT02599714).
Triple-negative BCBM
Patients with TN breast cancer (TNBC) also have a high risk of CNS failure, and those that do develop CNS metastasis have poor outcomes, with a median survival of 3–6 months [29, 65, 85, 94]. Treatment for these patients is limited by the lack of actionable targets for therapy, as well as the challenges posed by the BTB. A recent murine model demonstrated promising results using an amphiphilic polymer-lipid nanoparticle system to penetrate the BBB and deliver docetaxel to TNBC brain metastases in a murine model [50]. Treatment with the nanoparticle system significantly delayed tumor growth and prolonged survival. Another preclinical study demonstrated BBB penetration and improved outcomes with the combination of carboplatin and veliparib, a small molecule inhibitor of poly ADP-ribose polymerase [58]. Bevacizumab, the vascular endothelial growth factor (VEGF) inhibitor, has shown potential in the treatment of BCBM patients. Phase 2 trials, including TNBC, have demonstrated that bevacizumab can improve CNS response [67, 75]. There is an ongoing phase 2 trial of patients with recurrent or metastatic BRCA mutation associated and/or TNBC with or without brain metastases randomized to treatment with veliparib compared to placebo, both with concurrent cisplatin (NCT02595905) (Table 3).
Immune therapy
Antibody-directed therapies that alter interactions between CTLA-4 and PD-1/programmed death ligand 1 (PD-L1) have proven invaluable in the treatment of multiple cancers, including NSCLC, melanoma, and renal cell cancer [13, 82, 112]. The activity of the immune checkpoint inhibitors is not limited to extracranial disease, as trials have demonstrated significant response in the treatment of brain metastasis within melanoma and NSCLC [45, 133]. A number of clinical trials have also demonstrated that immune checkpoint inhibitors, such as pembrolizumab, nivolumab, and atezolizumab, can improve outcomes within breast cancer [2, 73, 86, 115]. Atezolizumab with nab-paclitaxel is now approved for use in PD-L1 positive locally advanced or metastatic TNBC not amenable to surgical resection [115]. The ability for immunotherapy to benefit patients with metastatic breast cancer, as well as the encouraging data on melanoma and NSCLC brain metastasis response, has sparked interest in investigating immune checkpoint inhibitors in the treatment of BCBM. In addition, studies have revealed constant immune surveillance of the CSF from meningeal lymph nodes with direct communication to the deep cervical nodes [74].
There has been significant interest in combining radiation therapy with immune checkpoint inhibition due to an immune priming effect noted from radiation therapy in preclinical studies noting upregulation of PD-L1 [32, 33] and enhanced tumor reduction and clinical data which have suggested clinical improvement including in the setting of brain metastases [3, 4, 108, 116, 135]. There are currently ongoing phase 2 trials examining the treatment of BCBM via SRS with concurrent Atezolizumab (NCT03483012), Nivolumab (NCT03807765), and Pembrolizumab (NCT03449238).
Conclusions
The significant heterogeneity of outcomes within BCBM implies opportunities to optimize precision treatment for these patients. While the treatment of BCBM has progressed greatly in the past decades, there are many ongoing efforts to improve clinical outcomes. This will continue to require a multidisciplinary approach improving our methods of BCBM screening, surgical and radiation delivery techniques as well as an improved understanding of the underlying biology of brain metastases to improve systemic treatment delivery. As systemic disease treatment continues to improve, simultaneous improvements in BCBM management will be required.
References
Adamo B, Deal AM, Burrows E, Geradts J, Hamilton E, Blackwell KL, Livasy C, Fritchie K, Prat A, Harrell JC, Ewend MG, Carey LA, Miller CR, Anders CK (2011) Phosphatidylinositol 3-kinase pathway activation in breast cancer brain metastases. Breast Cancer Res 13:R125. https://doi.org/10.1186/bcr3071
Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, Cescon DW, Iwata H, Campone M, Nanda R, Hui R, Curigliano G, Toppmeyer D, O’Shaughnessy J, Loi S, Paluch-Shimon S, Tan AR, Card D, Zhao J, Karantza V, Cortes J (2019) Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol 30:397–404. https://doi.org/10.1093/annonc/mdy517
Ahmed KA, Abuodeh YA, Echevarria MI, Arrington JA, Stallworth DG, Hogue C, Naghavi AO, Kim S, Kim Y, Patel BG, Sarangkasiri S, Johnstone PA, Sahebjam S, Khushalani NI, Forsyth PA, Harrison LB, Yu M, Etame AB, Caudell JJ (2016) Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy. Ann Oncol 27:2288–2294. https://doi.org/10.1093/annonc/mdw417
Ahmed KA, Kim S, Arrington J, Naghavi AO, Dilling TJ, Creelan BC, Antonia SJ, Caudell JJ, Harrison LB, Sahebjam S, Gray JE, Etame AB, Johnstone PA, Yu M, Perez BA (2017) Outcomes targeting the PD-1/PD-L1 axis in conjunction with stereotactic radiation for patients with non-small cell lung cancer brain metastases. J Neurooncol 133:331–338. https://doi.org/10.1007/s11060-017-2437-5
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672. https://doi.org/10.1016/S0140-6736(04)16250-8
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491. https://doi.org/10.1001/jama.295.21.2483
Arvold ND, Oh KS, Niemierko A, Taghian AG, Lin NU, Abi-Raad RF, Sreedhara M, Harris JR, Alexander BM (2012) Brain metastases after breast-conserving therapy and systemic therapy: incidence and characteristics by biologic subtype. Breast Cancer Res Treat 136:153–160. https://doi.org/10.1007/s10549-012-2243-x
Awada A, Colomer R, Inoue K, Bondarenko I, Badwe RA, Demetriou G, Lee SC, Mehta AO, Kim SB, Bachelot T, Goswami C, Deo S, Bose R, Wong A, Xu F, Yao B, Bryce R, Carey LA (2016) Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol 2:1557–1564. https://doi.org/10.1001/jamaoncol.2016.0237
Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, Jimenez M, Le Rhun E, Pierga JY, Goncalves A, Leheurteur M, Domont J, Gutierrez M, Cure H, Ferrero JM, Labbe-Devilliers C (2013) Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 14:64–71. https://doi.org/10.1016/S1470-2045(12)70432-1
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520–529. https://doi.org/10.1056/NEJMoa1109653
Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G, Ellis C, Florance A, Vukelja S, Bischoff J, Baselga J, O’Shaughnessy J (2012) Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol 30:2585–2592. https://doi.org/10.1200/JCO.2011.35.6725
Bonneau C, Paintaud G, Tredan O, Dubot C, Desvignes C, Dieras V, Taillibert S, Tresca P, Turbiez I, Li J, Passot C, Mefti F, Mouret-Fourme E, Le Rhun E, Gutierrez M (2018) Phase I feasibility study for intrathecal administration of trastuzumab in patients with HER2 positive breast carcinomatous meningitis. Eur J Cancer 95:75–84. https://doi.org/10.1016/j.ejca.2018.02.032
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639. https://doi.org/10.1056/NEJMoa1507643
Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massague J (2009) Genes that mediate breast cancer metastasis to the brain. Nature 459:1005–1009. https://doi.org/10.1038/nature08021
Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, Van Allen EM, Lawrence MS, Horowitz PM, Cibulskis K, Ligon KL, Tabernero J, Seoane J, Martinez-Saez E, Curry WT, Dunn IF, Paek SH, Park SH, McKenna A, Chevalier A, Rosenberg M, Barker FG 2nd, Gill CM, Van Hummelen P, Thorner AR, Johnson BE, Hoang MP, Choueiri TK, Signoretti S, Sougnez C, Rabin MS, Lin NU, Winer EP, Stemmer-Rachamimov A, Meyerson M, Garraway L, Gabriel S, Lander ES, Beroukhim R, Batchelor TT, Baselga J, Louis DN, Getz G, Hahn WC (2015) Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov 5:1164–1177. https://doi.org/10.1158/2159-8290.CD-15-0369
Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, Greenspoon J, Parney IF, Laack NNI, Ashman JB, Bahary JP, Hadjipanayis CG, Urbanic JJ, Barker FG 2nd, Farace E, Khuntia D, Giannini C, Buckner JC, Galanis E, Roberge D (2017) Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1049–1060. https://doi.org/10.1016/S1470-2045(17)30441-2
Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG 2nd, Deming R, Burri SH, Menard C, Chung C, Stieber VW, Pollock BE, Galanis E, Buckner JC, Asher AL (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 316:401–409. https://doi.org/10.1001/jama.2016.9839
Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, Choucair A, Fox S, Suh JH, Roberge D, Kavadi V, Bentzen SM, Mehta MP, Watkins-Bruner D, Radiation Therapy Oncology G (2013) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 15:1429–1437. https://doi.org/10.1093/neuonc/not114
Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, Tudor IC, Wang LI, Brammer MG, Shing M, Yood MU, Yardley DA (2011) Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res 17:4834–4843. https://doi.org/10.1158/1078-0432.CCR-10-2962
Saura EdA C, Hlauschek D, Oliveira M, Zardavas D, Jallitsch-Halper A, de la Pena L, Nuciforo P, Ballestrero A, Fornier MN, Boer K, Ciruelos E, Valero V, Wilson TR, Stout TJ, Hsu JY, Shi Y, Piccart M, Gnant M, Baselga J (2017) Preliminary results of LORELEI a phase II randomized double blind study of neoadjuvant letrozole (LET) plus taselisib versus LET plus placebo (PLA) in postmenopausal patients (pts) with ER-positive HER2-negative early breast cancer (EBC). Ann Oncol 28:v605–v649
Cagney DN, Lamba N, Sinha S, Catalano PJ, Bi WL, Alexander BM, Aizer AA (2019) Association of neurosurgical resection with development of pachymeningeal seeding in patients with brain metastases. JAMA Oncol 5:703–709. https://doi.org/10.1001/jamaoncol.2018.7204
Carey K, Anders ELR, Bachelot Thomas Denis, Yardley Denise A, Awada Ahmad, Conte Pier Franco, Kabos Peter, Bear Melissa, Yang Zhengyu, Chen Yanyun, Tolaney Sara M (2019) A phase II study of abemaciclib in patients (pts) with brain metastases (BM) secondary to HR + , HER2- metastatic breast cancer (MBC). J Clin Oncol 37:1017. https://doi.org/10.1200/JCO.2019.37.15_suppl.1017
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037–1044. https://doi.org/10.1016/S1470-2045(09)70263-3
Chowdhary M, Sen N, Chowdhary A, Usha L, Cobleigh MA, Wang D, Patel KR, Barry PN, Rao RD (2019) Safety and efficacy of Palbociclib and Radiation therapy in patients with metastatic breast cancer: initial results of a novel combination. Adv Radiat Oncol 4:453–457. https://doi.org/10.1016/j.adro.2019.03.011
Cortes J, Dieras V, Ro J, Barriere J, Bachelot T, Hurvitz S, Le Rhun E, Espie M, Kim SB, Schneeweiss A, Sohn JH, Nabholtz JM, Kellokumpu-Lehtinen PL, Taguchi J, Piacentini F, Ciruelos E, Bono P, Ould-Kaci M, Roux F, Joensuu H (2015) Afatinib alone or afatinib plus vinorelbine versus investigator’s choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): a randomised, open-label, multicentre, phase 2 trial. Lancet Oncol 16:1700–1710. https://doi.org/10.1016/S1470-2045(15)00373-3
Cortes J, Rugo HS, Awada A, Twelves C, Perez EA, Im SA, Gomez-Pardo P, Schwartzberg LS, Dieras V, Yardley DA, Potter DA, Mailliez A, Moreno-Aspitia A, Ahn JS, Zhao C, Hoch U, Tagliaferri M, Hannah AL, O’Shaughnessy J (2017) Prolonged survival in patients with breast cancer and a history of brain metastases: results of a preplanned subgroup analysis from the randomized phase III BEACON trial. Breast Cancer Res Treat 165:329–341. https://doi.org/10.1007/s10549-017-4304-7
Cristina Saura MO, Feng Yin-Hsun, Dai Ming-Shen, Hurvitz Sara A, Kim Sung-Bae, Moy Beverly, Delaloge Suzette, Gradishar William John, Masuda Norikazu, Palacova Marketa, Trudeau Maureen E, Mattson Johanna, Yap Yoon Sim, Bryce Richard, Yao Bin, Bebchuk Judith D, Keyvanjah Kiana, Brufsky Adam (2019) Neratinib + capecitabine versus lapatinib + capecitabine in patients with HER2 + metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: findings from the multinational, randomized, phase III NALA trial. JCO 37:1002. https://doi.org/10.1200/JCO.2019.37.15_suppl.1002
Dawood S, Broglio K, Esteva FJ, Ibrahim NK, Kau SW, Islam R, Aldape KD, Yu TK, Hortobagyi GN, Gonzalez-Angulo AM (2008) Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol 19:1242–1248. https://doi.org/10.1093/annonc/mdn036
Dawood S, Broglio K, Esteva FJ, Yang W, Kau SW, Islam R, Albarracin C, Yu TK, Green M, Hortobagyi GN, Gonzalez-Angulo AM (2009) Survival among women with triple receptor-negative breast cancer and brain metastases. Ann Oncol 20:621–627. https://doi.org/10.1093/annonc/mdn682
de Gooijer MC, Zhang P, Thota N, Mayayo-Peralta I, Buil LC, Beijnen JH, van Tellingen O (2015) P-glycoprotein and breast cancer resistance protein restrict the brain penetration of the CDK4/6 inhibitor palbociclib. Invest New Drugs 33:1012–1019. https://doi.org/10.1007/s10637-015-0266-y
Deeken JF, Loscher W (2007) The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res 13:1663–1674. https://doi.org/10.1158/1078-0432.CCR-06-2854
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX (2014) Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 124:687–695. https://doi.org/10.1172/JCI67313
Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S (2009) Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 15:5379–5388. https://doi.org/10.1158/1078-0432.CCR-09-0265
Di Leo A, Gomez HL, Aziz Z, Zvirbule Z, Bines J, Arbushites MC, Guerrera SF, Koehler M, Oliva C, Stein SH, Williams LS, Dering J, Finn RS, Press MF (2008) Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol 26:5544–5552. https://doi.org/10.1200/JCO.2008.16.2578
Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, van Dongen GA, Schroder CP, Lub-de Hooge MN, de Vries EG (2010) Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 87:586–592. https://doi.org/10.1038/clpt.2010.12
Figura NB, Potluri TK, Mohammadi H, Oliver DE, Arrington JA, Robinson TJ, Etame AB, Tran ND, Liu JK, Soliman H, Forsyth PA, Sahebjam S, Yu HM, Han HS, Ahmed KA (2019) CDK 4/6 inhibitors and stereotactic radiation in the management of hormone receptor positive breast cancer brain metastases. J Neurooncol 144:583–589. https://doi.org/10.1007/s11060-019-03260-6
Figura NB, Rizk VT, Mohammadi H, Evernden B, Mokhtari S, Yu HM, Robinson TJ, Etame AB, Tran ND, Liu J, Washington I, Diaz R, Czerniecki BJ, Soliman H, Han HS, Sahebjam S, Forsyth PA, Ahmed KA (2019) Clinical outcomes of breast leptomeningeal disease treated with intrathecal trastuzumab, intrathecal chemotherapy, or whole brain radiation therapy. Breast Cancer Res Treat 175:781–788. https://doi.org/10.1007/s10549-019-05170-7
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E, Lu DR, Randolph S, Dieras V, Slamon DJ (2016) Palbociclib and Letrozole in advanced breast cancer. N Engl J Med 375:1925–1936. https://doi.org/10.1056/NEJMoa1607303
Fitzgerald DP, Palmieri D, Hua E, Hargrave E, Herring JM, Qian Y, Vega-Valle E, Weil RJ, Stark AM, Vortmeyer AO, Steeg PS (2008) Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis 25:799–810. https://doi.org/10.1007/s10585-008-9193-z
Freedman RA, Gelman RS, Anders CK, Melisko ME, Parsons HA, Cropp AM, Silvestri K, Cotter CM, Componeschi KP, Marte JM, Connolly RM, Moy B, Van Poznak CH, Blackwell KL, Puhalla SL, Jankowitz RC, Smith KL, Ibrahim N, Moynihan TJ, O’Sullivan CC, Nangia J, Niravath P, Tung N, Pohlmann PR, Burns R, Rimawi MF, Krop IE, Wolff AC, Winer EP, Lin NU, Translational Breast Cancer Research C (2019) TBCRC 022: a Phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol 37:1081–1089. https://doi.org/10.1200/JCO.18.01511
Gabos Z, Sinha R, Hanson J, Chauhan N, Hugh J, Mackey JR, Abdulkarim B (2006) Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol 24:5658–5663. https://doi.org/10.1200/JCO.2006.07.0250
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37:745–751. https://doi.org/10.1016/s0360-3016(96)00619-0
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–2743. https://doi.org/10.1056/NEJMoa064320
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Park IH, Tredan O, Chen SC, Manso L, Freedman OC, Garnica Jaliffe G, Forrester T, Frenzel M, Barriga S, Smith IC, Bourayou N, Di Leo A (2017) MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35:3638–3646. https://doi.org/10.1200/JCO.2017.75.6155
Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, Tsiouris AJ, Cohen J, Vortmeyer A, Jilaveanu L, Yu J, Hegde U, Speaker S, Madura M, Ralabate A, Rivera A, Rowen E, Gerrish H, Yao X, Chiang V, Kluger HM (2016) Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 17:976–983. https://doi.org/10.1016/S1470-2045(16)30053-5
Gondi VDS, Brown PD, Wefel JS, Tome WA, Bruner DW, Bovi JA, Robinson CG, Khuntia D, Grosshans DR, Konski AA (2018) Preservation of neurocognitive function (NCF) with conformal avoidance of the hippocampus during whole-brain radiotherapy (HA-WBRT) for brain metastases: preliminary results of phase III trial NRG Oncology CC001. Int J Radiat Oncol Biol Phys 102:1607
Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN, Konski AA, Bauman GS, Shah S, Shi W, Wendland M, Kachnic L, Mehta MP (2014) Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 32:3810–3816. https://doi.org/10.1200/JCO.2014.57.2909
Hamilton E, Murthy R, Ferrario C, Conlin A, Krop I, Falkson C, Khan Q, Chamberlain M, Gray T, Borges V (2018) Abstract P5-20-01: prolonged progression-free survival (PFS) in advanced HER2 + metastatic breast cancer with or without brain metastases: a pooled analysis of tucatinib phase 1b studies
Hashizume R, Zhang A, Mueller S, Prados MD, Lulla RR, Goldman S, Saratsis AM, Mazar AP, Stegh AH, Cheng SY, Horbinski C, Haas-Kogan DA, Sarkaria JN, Waldman T, James CD (2016) Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro Oncol 18:1519–1528. https://doi.org/10.1093/neuonc/now106
He C, Cai P, Li J, Zhang T, Lin L, Abbasi AZ, Henderson JT, Rauth AM, Wu XY (2017) Blood-brain barrier-penetrating amphiphilic polymer nanoparticles deliver docetaxel for the treatment of brain metastases of triple negative breast cancer. J Control Release 246:98–109. https://doi.org/10.1016/j.jconrel.2016.12.019
Heitz F, Harter P, Lueck HJ, Fissler-Eckhoff A, Lorenz-Salehi F, Scheil-Bertram S, Traut A, du Bois A (2009) Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer 45:2792–2798. https://doi.org/10.1016/j.ejca.2009.06.027
Hicks DG, Short SM, Prescott NL, Tarr SM, Coleman KA, Yoder BJ, Crowe JP, Choueiri TK, Dawson AE, Budd GT, Tubbs RR, Casey G, Weil RJ (2006) Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Pathol 30:1097–1104. https://doi.org/10.1097/01.pas.0000213306.05811.b9
Hoefnagel LD, van de Vijver MJ, van Slooten HJ, Wesseling P, Wesseling J, Westenend PJ, Bart J, Seldenrijk CA, Nagtegaal ID, Oudejans J, van der Valk P, van der Groep P, de Vries EG, van der Wall E, van Diest PJ (2010) Receptor conversion in distant breast cancer metastases. Breast Cancer Res 12:R75. https://doi.org/10.1186/bcr2645
Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, Chow L, Sohn J, Lee KS, Campos-Gomez S, Villanueva-Vazquez R, Jung KH, Chakravartty A, Hughes G, Gounaris I, Rodriguez-Lorenc K, Taran T, Hurvitz S, Tripathy D (2019) Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 381:307–316. https://doi.org/10.1056/NEJMoa1903765
Ippen FM, Grosch JK, Subramanian M, Kuter BM, Liederer BM, Plise EG, Mora JL, Nayyar N, Schmidt SP, Giobbie-Hurder A, Martinez-Lage M, Carter SL, Cahill DP, Wakimoto H, Brastianos PK (2019) Targeting the PI3K/Akt/mTOR-pathway with the pan-Akt inhibitor GDC-0068 in PIK3CA-mutant breast cancer brain metastases. Neuro Oncol. https://doi.org/10.1093/neuonc/noz105
Jung J, Lee SH, Park M, Youn JH, Shin SH, Gwak HS, Yoo H (2018) Discordances in ER, PR, and HER2 between primary breast cancer and brain metastasis. J Neurooncol 137:295–302. https://doi.org/10.1007/s11060-017-2717-0
Kalkanis SN, Kondziolka D, Gaspar LE, Burri SH, Asher AL, Cobbs CS, Ammirati M, Robinson PD, Andrews DW, Loeffler JS, McDermott M, Mehta MP, Mikkelsen T, Olson JJ, Paleologos NA, Patchell RA, Ryken TC, Linskey ME (2010) The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96:33–43. https://doi.org/10.1007/s11060-009-0061-8
Karginova O, Siegel MB, Van Swearingen AE, Deal AM, Adamo B, Sambade MJ, Bazyar S, Nikolaishvili-Feinberg N, Bash R, O’Neal S, Sandison K, Parker JS, Santos C, Darr D, Zamboni W, Lee YZ, Miller CR, Anders CK (2015) Efficacy of carboplatin alone and in combination with ABT888 in intracranial murine models of BRCA-mutated and BRCA-wild-type triple-negative breast cancer. Mol Cancer Ther 14:920–930. https://doi.org/10.1158/1535-7163.MCT-14-0474
Kayama T, Sato S, Sakurada K, Mizusawa J, Nishikawa R, Narita Y, Sumi M, Miyakita Y, Kumabe T, Sonoda Y, Arakawa Y, Miyamoto S, Beppu T, Sugiyama K, Nakamura H, Nagane M, Nakasu Y, Hashimoto N, Terasaki M, Matsumura A, Ishikawa E, Wakabayashi T, Iwadate Y, Ohue S, Kobayashi H, Kinoshita M, Asano K, Mukasa A, Tanaka K, Asai A, Nakamura H, Abe T, Muragaki Y, Iwasaki K, Aoki T, Watanabe T, Sasaki H, Izumoto S, Mizoguchi M, Matsuo T, Takeshima H, Hayashi M, Jokura H, Mizowaki T, Shimizu E, Shirato H, Tago M, Katayama H, Fukuda H, Shibui S, Japan Clinical Oncology G (2018) Effects of surgery with salvage stereotactic radiosurgery versus surgery with whole-brain radiation therapy in patients with one to four brain metastases (JCOG0504): a phase III, noninferiority, randomized controlled trial. J Clin Oncol. https://doi.org/10.1200/JCO.2018.78.6186
Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28:3271–3277. https://doi.org/10.1200/JCO.2009.25.9820
Khaitan D, Sankpal UT, Weksler B, Meister EA, Romero IA, Couraud PO, Ningaraj NS (2009) Role of KCNMA1 gene in breast cancer invasion and metastasis to brain. BMC Cancer 9:258. https://doi.org/10.1186/1471-2407-9-258
Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 29:134–141. https://doi.org/10.1200/JCO.2010.30.1655
Krop IE, Lin NU, Blackwell K, Guardino E, Huober J, Lu M, Miles D, Samant M, Welslau M, Dieras V (2015) Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol 26:113–119. https://doi.org/10.1093/annonc/mdu486
Kumthekar PGW, Lin N, Pentsova E, Groves M, Jeyapalan S, Melisko M, Grimm S, Lassman AB, Raizer J (2018) Intrathecal (IT) traztuzumab (T) for the treatment of leptomeningeal metastaSES (LM) in patients (PTS) with human epidermal growth factor receptor 2-positive (HER2 +) cancer: a multicenter phase 1/2 study. Neuro Oncol 20:58. https://doi.org/10.1093/neuonc/noy148.234
Leone JP, Leone J, Zwenger AO, Iturbe J, Leone BA, Vallejo CT (2017) Prognostic factors and survival according to tumour subtype in women presenting with breast cancer brain metastases at initial diagnosis. Eur J Cancer 74:17–25. https://doi.org/10.1016/j.ejca.2016.12.015
Lien EA, Wester K, Lonning PE, Solheim E, Ueland PM (1991) Distribution of tamoxifen and metabolites into brain tissue and brain metastases in breast cancer patients. Br J Cancer 63:641–645. https://doi.org/10.1038/bjc.1991.147
Lin NG, Younger WJ, Sohl J, Freedman RA, Sorensen G, Bullitt E, Harris GJ, Morganstern D, Schneider BP, Krop IE, Winer EP (2013) Phase II trial of carboplatin (C) and bevacizumab (BEV) in patients (pts) with breast cancer brain metastases (BCBM). JCO 31:513. https://doi.org/10.1200/jco.2013.31.15_suppl.513
Lin NU, Freedman RA, Ramakrishna N, Younger J, Storniolo AM, Bellon JR, Come SE, Gelman RS, Harris GJ, Henderson MA, Macdonald SM, Mahadevan A, Eisenberg E, Ligibel JA, Mayer EL, Moy B, Eichler AF, Winer EP (2013) A phase I study of lapatinib with whole brain radiotherapy in patients with Human Epidermal Growth Factor Receptor 2 (HER2)-positive breast cancer brain metastases. Breast Cancer Res Treat 142:405–414. https://doi.org/10.1007/s10549-013-2754-0
Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, Bendszus M, Brown PD, Camidge DR, Chang SM, Dancey J, de Vries EG, Gaspar LE, Harris GJ, Hodi FS, Kalkanis SN, Linskey ME, Macdonald DR, Margolin K, Mehta MP, Schiff D, Soffietti R, Suh JH, van den Bent MJ, Vogelbaum MA, Wen PY, Response Assessment in Neuro-Oncology (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16:e270–e278. https://doi.org/10.1016/S1470-2045(15)70057-4
Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, Adkins CE, Roberts A, Thorsheim HR, Gaasch JA, Huang S, Palmieri D, Steeg PS, Smith QR (2010) Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 16:5664–5678. https://doi.org/10.1158/1078-0432.CCR-10-1564
Lohmann P, Kocher M, Ceccon G, Bauer EK, Stoffels G, Viswanathan S, Ruge MI, Neumaier B, Shah NJ, Fink GR, Langen KJ, Galldiks N (2018) Combined FET PET/MRI radiomics differentiates radiation injury from recurrent brain metastasis. Neuroimage Clin 20:537–542. https://doi.org/10.1016/j.nicl.2018.08.024
Lohmann P, Stoffels G, Ceccon G, Rapp M, Sabel M, Filss CP, Kamp MA, Stegmayr C, Neumaier B, Shah NJ, Langen KJ, Galldiks N (2017) Radiation injury vs. recurrent brain metastasis: combining textural feature radiomics analysis and standard parameters may increase (18)F-FET PET accuracy without dynamic scans. Eur Radiol 27:2916–2927. https://doi.org/10.1007/s00330-016-4638-2
Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, Campone M, Biganzoli L, Bonnefoi H, Jerusalem G, Bartsch R, Rabaglio-Poretti M, Kammler R, Maibach R, Smyth MJ, Di Leo A, Colleoni M, Viale G, Regan MM, Andre F, International Breast Cancer Study G, the Breast International G (2019) Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol 20:371–382. https://doi.org/10.1016/S1470-2045(18)30812-X
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–341. https://doi.org/10.1038/nature14432
Lu YS, Chen TW, Lin CH, Yeh DC, Tseng LM, Wu PF, Rau KM, Chen BB, Chao TC, Huang SM, Huang CS, Shih TT, Cheng AL, Taiwan Breast Cancer C (2015) Bevacizumab preconditioning followed by Etoposide and Cisplatin is highly effective in treating brain metastases of breast cancer progressing from whole-brain radiotherapy. Clin Cancer Res 21:1851–1858. https://doi.org/10.1158/1078-0432.CCR-14-2075
Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P, Settle S, Prabhu SS, Lang FF, Levine N, McGovern S, Sulman E, McCutcheon IE, Azeem S, Cahill D, Tatsui C, Heimberger AB, Ferguson S, Ghia A, Demonte F, Raza S, Guha-Thakurta N, Yang J, Sawaya R, Hess KR, Rao G (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1040–1048. https://doi.org/10.1016/S1470-2045(17)30414-X
Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, Claus EB, Lee EQ, Wen PY, Haas-Kogan DA, Alexander BM, Lin NU, Aizer AA (2017) Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol 3:1069–1077. https://doi.org/10.1001/jamaoncol.2017.0001
Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H, von Minckwitz G, Chia SKL, Mansi J, Barrios CH, Gnant M, Tomasevic Z, Denduluri N, Separovic R, Gokmen E, Bashford A, Ruiz Borrego M, Kim SB, Jakobsen EH, Ciceniene A, Inoue K, Overkamp F, Heijns JB, Armstrong AC, Link JS, Joy AA, Bryce R, Wong A, Moran S, Yao B, Xu F, Auerbach A, Buyse M, Chan A, Exte NETSG (2017) Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 18:1688–1700. https://doi.org/10.1016/S1470-2045(17)30717-9
McGuire WL, Horwitz KB, Pearson OH, Segaloff A (1977) Current status of estrogen and progesterone receptors in breast cancer. Cancer 39:2934–2947
Mintz AH, Kestle J, Rathbone MP, Gaspar L, Hugenholtz H, Fisher B, Duncan G, Skingley P, Foster G, Levine M (1996) A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 78:1470–1476
Morikawa A, Peereboom DM, Thorsheim HR, Samala R, Balyan R, Murphy CG, Lockman PR, Simmons A, Weil RJ, Tabar V, Steeg PS, Smith QR, Seidman AD (2015) Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: a prospective study. Neuro Oncol 17:289–295. https://doi.org/10.1093/neuonc/nou141
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, CheckMate I (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803–1813. https://doi.org/10.1056/NEJMoa1510665
Moulder S, Hamilton E, Ferrario C, Conlin A, Krop I, Chamberlain M, Gray T, Borges V (2017) 264PProgression-free survival (PFS) and site of first progression in HER2 + metastatic breast cancer (MBC) patients (pts) with (w) or without (w/o) brain metastases: a pooled analysis of tucatinib phase I studies. Ann Oncol. https://doi.org/10.1093/annonc/mdx365.027
Murthy RK, Hamilton EP, Ferrario C, Aucoin N, Falkson CI, Chamberlain MC, Gray T, Borges VF (2018) Clinical benefit of tucatinib after isolated brain progression: A retrospective pooled analysis of tucatinib phase 1b studies in HER2 + breast cancer. J Clin Oncol 36:1015
Nam BH, Kim SY, Han HS, Kwon Y, Lee KS, Kim TH, Ro J (2008) Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res 10:R20. https://doi.org/10.1186/bcr1870
Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, Karantza V, Buisseret L (2016) Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 Study. J Clin Oncol 34:2460–2467. https://doi.org/10.1200/JCO.2015.64.8931
National Comprehensive Cancer N Breast Cancer Version 3.2019
National Comprehensive Cancer N Cutaneous Melanoma Version 2.2019
National Comprehensive Cancer N Non-Small Cell Lung Cancer Version 7.2019
National Comprehensive Cancer N Small Cell Lung Cancer Version 1.2020
Nduom EK, Yang C, Merrill MJ, Zhuang Z, Lonser RR (2013) Characterization of the blood-brain barrier of metastatic and primary malignant neoplasms. J Neurosurg 119:427–433. https://doi.org/10.3171/2013.3.JNS122226
Neman J, Termini J, Wilczynski S, Vaidehi N, Choy C, Kowolik CM, Li H, Hambrecht AC, Roberts E, Jandial R (2014) Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc Natl Acad Sci USA 111:984–989. https://doi.org/10.1073/pnas.1322098111
Nieder C, Spanne O, Mehta MP, Grosu AL, Geinitz H (2011) Presentation, patterns of care, and survival in patients with brain metastases: what has changed in the last 20 years? Cancer 117:2505–2512. https://doi.org/10.1002/cncr.25707
Niikura N, Hayashi N, Masuda N, Takashima S, Nakamura R, Watanabe K, Kanbayashi C, Ishida M, Hozumi Y, Tsuneizumi M, Kondo N, Naito Y, Honda Y, Matsui A, Fujisawa T, Oshitanai R, Yasojima H, Tokuda Y, Saji S, Iwata H (2014) Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Cancer Res Treat 147:103–112. https://doi.org/10.1007/s10549-014-3090-8
Okada H, Pollack IF (2011) Do we need novel radiologic response criteria for brain tumor immunotherapy? Expert Rev Neurother 11:619–622. https://doi.org/10.1586/ern.11.49
Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, Ellingson BM, Hashimoto N, Pollack IF, Brandes AA, Franceschi E, Herold-Mende C, Nayak L, Panigrahy A, Pope WB, Prins R, Sampson JH, Wen PY, Reardon DA (2015) Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 16:e534–e542. https://doi.org/10.1016/S1470-2045(15)00088-1
Osborne CK, Schiff R (2011) Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 62:233–247. https://doi.org/10.1146/annurev-med-070909-182917
Park EJ, Zhang YZ, Vykhodtseva N, McDannold N (2012) Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Control Release 163:277–284. https://doi.org/10.1016/j.jconrel.2012.09.007
Park YH, Park MJ, Ji SH, Yi SY, Lim DH, Nam DH, Lee JI, Park W, Choi DH, Huh SJ, Ahn JS, Kang WK, Park K, Im YH (2009) Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer 100:894–900. https://doi.org/10.1038/sj.bjc.6604941
Parvez K, Parvez A, Zadeh G (2014) The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci 15:11832–11846. https://doi.org/10.3390/ijms150711832
Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, Markesbery WR, Foon KA, Young B (1998) Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 280:1485–1489. https://doi.org/10.1001/jama.280.17.1485
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494–500. https://doi.org/10.1056/NEJM199002223220802
Patel KR, Burri SH, Asher AL, Crocker IR, Fraser RW, Zhang C, Chen Z, Kandula S, Zhong J, Press RH, Olson JJ, Oyesiku NM, Wait SD, Curran WJ, Shu HK, Prabhu RS (2016) Comparing preoperative with postoperative stereotactic radiosurgery for resectable brain metastases: a multi-institutional analysis. Neurosurgery 79:279–285. https://doi.org/10.1227/NEU.0000000000001096
Perez EA, Awada A, O’Shaughnessy J, Rugo HS, Twelves C, Im SA, Gomez-Pardo P, Schwartzberg LS, Dieras V, Yardley DA, Potter DA, Mailliez A, Moreno-Aspitia A, Ahn JS, Zhao C, Hoch U, Tagliaferri M, Hannah AL, Cortes J (2015) Etirinotecan pegol (NKTR-102) versus treatment of physician’s choice in women with advanced breast cancer previously treated with an anthracycline, a taxane, and capecitabine (BEACON): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 16:1556–1568. https://doi.org/10.1016/S1470-2045(15)00332-0
Prabhu RS, Turner BE, Asher AL, Marcrom SR, Fiveash JB, Foreman PM, Press RH, Patel KR, Curran WJ, Breen WG, Brown PD, Jethwa KR, Grills IS, Arden JD, Foster LM, Manning MA, Stern JD, Soltys SG, Burri SH (2019) A multi-institutional analysis of presentation and outcomes for leptomeningeal disease recurrence after surgical resection and radiosurgery for brain metastases. Neuro Oncol. https://doi.org/10.1093/neuonc/noz049
Preusser M, De Mattos-Arruda L, Thill M, Criscitiello C, Bartsch R, Ruhstaller T, de Azambuja E, Zielinski CC (2018) CDK4/6 inhibitors in the treatment of patients with breast cancer: summary of a multidisciplinary round-table discussion. ESMO Open 3:e000368. https://doi.org/10.1136/esmoopen-2018-000368
Pukrop T, Dehghani F, Chuang HN, Lohaus R, Bayanga K, Heermann S, Regen T, Van Rossum D, Klemm F, Schulz M, Siam L, Hoffmann A, Trumper L, Stadelmann C, Bechmann I, Hanisch UK, Binder C (2010) Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia 58:1477–1489. https://doi.org/10.1002/glia.21022
Qian JM, Yu JB, Kluger HM, Chiang VL (2016) Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer 122:3051–3058. https://doi.org/10.1002/cncr.30138
Raub TJ, Wishart GN, Kulanthaivel P, Staton BA, Ajamie RT, Sawada GA, Gelbert LM, Shannon HE, Sanchez-Martinez C, De Dios A (2015) Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metab Dispos 43:1360–1371. https://doi.org/10.1124/dmd.114.062745
Reese TS, Karnovsky MJ (1967) Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol 34:207–217. https://doi.org/10.1083/jcb.34.1.207
Rimawi MF, Aleixo SB, Rozas AA, de Matos Nunes, Neto J, Caleffi M, Figueira AC, Souza SC, Reiriz AB, Gutierrez C, Arantes H, Uttenreuther-Fischer MM, Solca F, Osborne CK (2015) A neoadjuvant, randomized, open-label phase II trial of afatinib versus trastuzumab versus lapatinib in patients with locally advanced HER2-positive breast cancer. Clin Breast Cancer 15:101–109. https://doi.org/10.1016/j.clbc.2014.11.004
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372:320–330. https://doi.org/10.1056/NEJMoa1412082
Rostami R, Mittal S, Rostami P, Tavassoli F, Jabbari B (2016) Brain metastasis in breast cancer: a comprehensive literature review. J Neurooncol 127:407–414. https://doi.org/10.1007/s11060-016-2075-3
Sahgal A, Aoyama H, Kocher M, Neupane B, Collette S, Tago M, Shaw P, Beyene J, Chang EL (2015) Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 91:710–717. https://doi.org/10.1016/j.ijrobp.2014.10.024
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA, Investigators IMT (2018) Atezolizumab and Nab-Paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108–2121. https://doi.org/10.1056/NEJMoa1809615
Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, Garon EB, Lee P (2017) Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 18:895–903. https://doi.org/10.1016/S1470-2045(17)30380-7
Shen Q, Sahin AA, Hess KR, Suki D, Aldape KD, Sawaya R, Ibrahim NK (2015) Breast cancer with brain metastases: clinicopathologic features, survival, and paired biomarker analysis. Oncologist 20:466–473. https://doi.org/10.1634/theoncologist.2014-0107
Sirkisoon SR, Carpenter RL, Rimkus T, Doheny D, Zhu D, Aguayo NR, Xing F, Chan M, Ruiz J, Metheny-Barlow LJ, Strowd R, Lin J, Regua AT, Arrigo A, Anguelov M, Pasche B, Debinski W, Watabe K, Lo HW (2019) TGLI1 transcription factor mediates breast cancer brain metastasis via activating metastasis-initiating cancer stem cells and astrocytes in the tumor microenvironment. Oncogene. https://doi.org/10.1038/s41388-019-0959-3
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, Koh H, Grischke EM, Frenzel M, Lin Y, Barriga S, Smith IC, Bourayou N, Llombart-Cussac A (2017) MONARCH 2: abemaciclib in combination with fulvestrant in women with HR +/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35:2875–2884. https://doi.org/10.1200/JCO.2017.73.7585
Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, Kros JM, Marosi C, Metellus P, Radbruch A, Villa Freixa SS, Brada M, Carapella CM, Preusser M, Le Rhun E, Ruda R, Tonn JC, Weber DC, Weller M (2017) Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol 19:162–174. https://doi.org/10.1093/neuonc/now241
Soni A, Ren Z, Hameed O, Chanda D, Morgan CJ, Siegal GP, Wei S (2015) Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol 143:471–478. https://doi.org/10.1309/AJCPYO5FSV3UPEXS
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W (2008) A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 70:510–514. https://doi.org/10.1016/j.ijrobp.2007.06.074
Sperduto PW, Shanley R, Luo X, Andrews D, Werner-Wasik M, Valicenti R, Bahary JP, Souhami L, Won M, Mehta M (2014) Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1-3 brain metastases; poststratified by the graded prognostic assessment (GPA). Int J Radiat Oncol Biol Phys 90:526–531. https://doi.org/10.1016/j.ijrobp.2014.07.002
Stark AM, Anuszkiewicz B, Mentlein R, Yoneda T, Mehdorn HM, Held-Feindt J (2007) Differential expression of matrix metalloproteinases in brain- and bone-seeking clones of metastatic MDA-MB-231 breast cancer cells. J Neurooncol 81:39–48. https://doi.org/10.1007/s11060-006-9207-0
Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V (2007) Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs 18:23–28. https://doi.org/10.1097/01.cad.0000236313.50833.ee
Stumpf PK, Cittelly DM, Robin TP, Carlson JA, Stuhr KA, Contreras-Zarate MJ, Lai S, Ormond DR, Rusthoven CG, Gaspar LE, Rabinovitch R, Kavanagh BD, Liu A, Diamond JR, Kabos P, Fisher CM (2019) Combination of Trastuzumab emtansine and stereotactic radiosurgery results in high rates of clinically significant radionecrosis and dysregulation of aquaporin-4. Clin Cancer Res 25:3946–3953. https://doi.org/10.1158/1078-0432.CCR-18-2851
Subbiah IM, Lei X, Weinberg JS, Sulman EP, Chavez-MacGregor M, Tripathy D, Gupta R, Varma A, Chouhan J, Guevarra RP, Valero V, Gilbert MR, Gonzalez-Angulo AM (2015) Validation and development of a modified breast graded prognostic assessment as a tool for survival in patients with breast cancer and brain metastases. J Clin Oncol 33:2239–2245. https://doi.org/10.1200/JCO.2014.58.8517
Swain SM, Baselga J, Miles D, Im YH, Quah C, Lee LF, Cortes J (2014) Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol 25:1116–1121. https://doi.org/10.1093/annonc/mdu133
Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Knott A, Clark E, Ross G, Benyunes MC, Baselga J (2013) Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 14:461–471. https://doi.org/10.1016/S1470-2045(13)70130-X
Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Goncalves A (2012) Recent trends in epidemiology of brain metastases: an overview. Anticancer Res 32:4655–4662
Tamura K, Kurihara H, Yonemori K, Tsuda H, Suzuki J, Kono Y, Honda N, Kodaira M, Yamamoto H, Yunokawa M, Shimizu C, Hasegawa K, Kanayama Y, Nozaki S, Kinoshita T, Wada Y, Tazawa S, Takahashi K, Watanabe Y, Fujiwara Y (2013) 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med 54:1869–1875. https://doi.org/10.2967/jnumed.112.118612
Taskar KS, Rudraraju V, Mittapalli RK, Samala R, Thorsheim HR, Lockman J, Gril B, Hua E, Palmieri D, Polli JW, Castellino S, Rubin SD, Lockman PR, Steeg PS, Smith QR (2012) Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm Res 29:770–781. https://doi.org/10.1007/s11095-011-0601-8
Tawbi HA FP, Hodi FS, Lao CD, Moschos SJ, Hamid O, Atkins MB, Lewis KD, Thomas RP, Glaspy JA, Jang S (2019) Efficacy and safety of the combination of nivolumab (NIVO) plus ipilimumab (IPI) in patients with symptomatic melanoma brain metastases (CheckMate 204). J Clin Oncol
Termini J, Neman J, Jandial R (2014) Role of the neural niche in brain metastatic cancer. Cancer Res 74:4011–4015. https://doi.org/10.1158/0008-5472.CAN-14-1226
Theelen W, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts J, Dumoulin DW, Bahce I, Niemeijer AN, de Langen AJ, Monkhorst K, Baas P (2019) Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.1478
Tian X, Nyberg S, Madsen J, Daneshpour N, Armes SP, Berwick J, Azzouz M, Shaw P, Abbott NJ, Battaglia G (2015) LRP-1-mediated intracellular antibody delivery to the Central Nervous System. Sci Rep 5:11990. https://doi.org/10.1038/srep11990
Tolaney SS, Le Rhun E, Lin NU, Bear MM, Yang Z, Chen Y, Anders CK (2019) Abstract P1-19-01: a phase 2 study of abemaciclib in patients with leptomeningeal metastases secondary to HR + , HER2- breast cancer. Am Assoc Cancer Res. https://doi.org/10.1158/1538-7445.SABCS18-P1-19-01
Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Andre F, Puyana Theall K, Huang X, Giorgetti C, Huang Bartlett C, Cristofanilli M (2018) Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 379:1926–1936. https://doi.org/10.1056/NEJMoa1810527
Van Swearingen AE, Siegel MB, Anders CK (2014) Breast cancer brain metastases: evidence for neuronal-like adaptation in a ‘breast-to-brain’ transition? Breast Cancer Res 16:304. https://doi.org/10.1186/bcr3651
Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorff AR et al (1993) Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 33:583–590. https://doi.org/10.1002/ana.410330605
Verma N, Cowperthwaite MC, Burnett MG, Markey MK (2013) Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol 15:515–534. https://doi.org/10.1093/neuonc/nos307
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Dieras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783–1791. https://doi.org/10.1056/NEJMoa1209124
Whiteside TL (2008) The tumor microenvironment and its role in promoting tumor growth. Oncogene 27:5904–5912. https://doi.org/10.1038/onc.2008.271
Whittaker S, Madani D, Joshi S, Chung SA, Johns T, Day B, Khasraw M, McDonald KL (2017) Combination of palbociclib and radiotherapy for glioblastoma. Cell Death Discov 3:17033. https://doi.org/10.1038/cddiscovery.2017.33
Witzel I, Kantelhardt EJ, Milde-Langosch K, Ihnen M, Zeitz J, Harbeck N, Janicke F, Muller V (2011) Management of patients with brain metastases receiving trastuzumab treatment for metastatic breast cancer. Onkologie 34:304–308. https://doi.org/10.1159/000328679
Witzel I, Oliveira-Ferrer L, Pantel K, Muller V, Wikman H (2016) Breast cancer brain metastases: biology and new clinical perspectives. Breast Cancer Res 18:8. https://doi.org/10.1186/s13058-015-0665-1
Xing F, Liu Y, Sharma S, Wu K, Chan MD, Lo HW, Carpenter RL, Metheny-Barlow LJ, Zhou X, Qasem SA, Pasche B, Watabe K (2016) Activation of the c-Met pathway mobilizes an inflammatory network in the brain microenvironment to promote brain metastasis of breast cancer. Cancer Res 76:4970–4980. https://doi.org/10.1158/0008-5472.CAN-15-3541
Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, Yamanaka K, Sato Y, Jokura H, Yomo S, Nagano O, Kenai H, Moriki A, Suzuki S, Kida Y, Iwai Y, Hayashi M, Onishi H, Gondo M, Sato M, Akimitsu T, Kubo K, Kikuchi Y, Shibasaki T, Goto T, Takanashi M, Mori Y, Takakura K, Saeki N, Kunieda E, Aoyama H, Momoshima S, Tsuchiya K (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15:387–395. https://doi.org/10.1016/S1470-2045(14)70061-0
Yang H, Lee HW, Kim Y, Lee Y, Choi YS, Kim KH, Jin J, Lee J, Joo KM, Nam DH (2013) Radiosensitization of brain metastasis by targeting c-MET. Lab Invest 93:344–353. https://doi.org/10.1038/labinvest.2012.180
Zeng Q, Michael IP, Zhang P, Saghafinia S, Knott G, Jiao W, McCabe BD, Galvan JA, Robinson HPC, Zlobec I, Ciriello G, Hanahan D (2019) Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573:526–531. https://doi.org/10.1038/s41586-019-1576-6
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Hsiang-Hsuan Michael Yu has received spearker’s honoraria from BrainLab and is on the advisory boards of Novocure and Abbvie. Michael A. Vogelbaum has indirect equity and royalty interests in Infuseon Therapeutics, Inc. and has received honoraria from Tocagen, Inc. and Celgene. Hatem Soliman serves as a consultant for Astrazeneca, Celgene, Novartis, PUMA, and Eisai. Brian J. Czerniecki has intellectual property on a HER2 dendritic cell vaccine. Peter A. Forsyth has received research funding from Pfizer and Celgene and is on the advisory boards of Novocure, BTG, Inovio, AbbVie, Ziopharm, Tocagen, and Pfizer. Hyo S. Han declares that she has received a speaker’s honorarium from Lilly Pharmaceuticals, research funding to the institution from Abbvie, Tesaro, TapImmune, Novartis, Bristol-Myers Squibb, Pfizer, SeattleGenetics, Prescient, Horizon, and Karyopharm. Kamran A. Ahmed has received research funding from Bristol-Myers Squibb and Genentech.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mills, M.N., Figura, N.B., Arrington, J.A. et al. Management of brain metastases in breast cancer: a review of current practices and emerging treatments. Breast Cancer Res Treat 180, 279–300 (2020). https://doi.org/10.1007/s10549-020-05552-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05552-2