Abstract
Purpose
To explore the ability of intraoperative specimen radiography (SR) to correctly identify positive margins in patients receiving breast conserving surgery (BCS). To assess whether the reoperation rate can be reduced by using this method.
Methods
This retrospective study included 470 consecutive cases receiving BCS due to a primarily diagnosed breast cancer. SR was carried out in two planes, assessing the specimen regarding the presence of the lesion and its relation to all margins. If indicated, re-excision of selective orientations was advised. Under consideration of gross inspection and the SR-findings, it was up to the surgeon whether to perform re-resections. The recommendations for re-excision were, separately for each orientation, compared to the histopathological results, serving as gold standard.
Results
Intraoperative SR was performed in 470 cases, thus 2820 margins were assessed. Of those, 2510 (89.0%) were negative and 310 (11.0%) positive. SR identified 2179 (77.3%) margins correctly as negative, whereas 331 (11.7%) clear margins were misjudged as positive. Of 310 infiltrated margins, SR identified 114 (4.0%) correctly, whereas 196 (7.0%) infiltrated margins were missed. This resulted in a sensitivity/specificity of 36.8%/86.8% and PPV/NPV of 25.6%/91.8%. Through targeted re-resections positive margins could be reduced by 31.0% [310 to 214 (7.6%)]. On case level, the rate of secondary procedures could be reduced by 37.0% [from 162 to 102 (21.7%)].
Conclusions
SR is a helpful tool to identify infiltrated margins and to reduce the rate of secondary surgeries by recommending targeted re-excisions of according orientations in order to obtain a final negative margin status.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the introduction of comprehensive mammography screening programs, malignant breast lesions are more likely to be detected early at an impalpable stage. The therapy of choice for most of these cases is breast conserving therapy (BCT), defined as the combination of breast conserving surgery (BCS) and adjuvant irradiation. Although significantly less tissue is removed, overall survival has been described as equal [1, 2] or superior [3, 4] in comparison to mastectomy. Furthermore, BCT is related to a higher quality of life [5, 6], a reduced risk of postoperative complications, and an improved body image [7], as well as a better patient satisfaction with the aesthetic outcome [8]. It is well known that higher specimen weight is one of the main risk factors for poor aesthetic outcome [9,10,11]. Consequently, improved aesthetical outcome is characterized by lower resected specimen weight [9].
An important complication of BCS are positive (= infiltrated) resection margins, a known prognostic factor for local recurrence [12, 13]. Hence, the goal of the primary surgery is to obtain tumor-free margins adhering to oncologic principles while resecting as little tissue as possible to reach an ideal aesthetic outcome. To achieve this, intraoperative margin assessment tools are used to identify infiltrated margins within the primary procedure, so immediate re-resections can be performed to obtain a final clear margin status and to prevent secondary re-resections, which increase the risk of poor satisfaction regarding the aesthetic result [14]. Whereas there are multiple techniques of intraoperative margin assessment, e.g., intraoperative ultrasound [15], breast imprint and scrape cytology [16], frozen section analysis [17] or MarginProbe [18], all employed with varying results, specimen radiography (SR) using two plane mammography and/or ultrasound is the widely used standard method. This study’s aim is to explore how accurately SR is able to assess all six of the specimens’ margins and if patients benefit of its use and the subsequent targeted re-resections as performed in our center.
Materials and methods
This study was approved by the ethics committee of Heidelberg University’s medical faculty.
Patient population
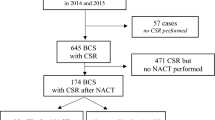
A total of 702 primary breast conserving surgeries (BCS) in 671 consecutive patients were performed due to a histologically confirmed malignant breast lesion between January 2014 and December 2015 at the Heidelberg University breast unit. Several patients had either bilateral (n = 23) or multicentric breast cancers (n = 8) and in this respect two lesions that were treated with BCS. Cases that did not receive SR (n = 57), mostly for reasons of palpability, were excluded from further analysis, as well as 175 patients who received neoadjuvant therapy. Accordingly, 470 two-view specimen radiographs form the basis of this retrospective consecutive analysis. Considered were all histological subtypes including ductal carcinoma in situ (DCIS), disregarding their palpability, tumor size, or execution of preoperative localization.
Specimen radiography and surgical procedure
Preoperatively, patients generally underwent ultrasound- or mammography-guided wire localization. Reasons for renunciation were close localization to the skin and/or good palpability. The position of the wire was controlled by mammography.
In the operating room, the tumorous tissue was excised and immediately marked by sutures on three surfaces (cranial, medial, and lateral) to ensure orientation. The specimens were then sent to the breast unit where they were oriented and positioned on the mammography plate, the simplified illustration of which is shown in Fig. 1.
Using Mammomat Inspiration (Siemens AG, Erlangen, Germany) with 1.4 × direct magnification, the first radiographs were generated. The specimens were then rotated 90 degrees to obtain the second view (Fig. 2). The specimen radiographs were, in comparison to the preoperative mammogram, reviewed by an experienced examiner concerning the presence of the malignant lesion and its relation to the resection margins. A margin was considered positive if the malignant lesion seemed to infiltrate the margin. In the event of poor visibility, ACUSON S2000 or S3000 ultrasound unit (SIEMENS Medical Solutions, Mountain View, CA, US), 18 MHz devices, were available for additional sonographic examination. A recommendation for re-excision of the according orientation was given directly to the surgeon if the examiner considered one or more of the resection margins to be infiltrated. It was up to the surgeon’s discretion if an extension of excision was to be performed. It shall be noted that not only SR but also the surgeon’s clinical impression after gross inspection served as a margin assessment tool and may have led to re-resections during the primary surgery even if SR did not recommend further excision. After the procedure, all specimens were sent to pathology for histological assessment, functioning as reference standard.
The pathologic staging included gross and microscopic inspection of all resection margins. According to the AJCC Cancer Staging Manual, the presence or absence of residual tumor was classified as R0 (No residual tumor), R1 (Microscopic residual tumor), R2 (Macroscopic residual tumor), or RX (Presence of residual tumor cannot be assessed) [19]. All margins with an assessment other than R0 were handled as positive. At the time of interest, R0 was defined as margin width ≥ 1 mm in invasive cancer and ≥ 2 mm in DCIS [20]. The pathologists were unaware of SR-findings. All examiners had access to clinical information concerning the respective patient.
To evaluate the diagnostic reliability, the SR-report was compared to the histopathological assessment of the specimen’s initial margins. Positive final resection margins set the indication for further surgical therapy.
Statistical analysis
Statistical analysis was carried out in co-operation with an independent statistician using the validated software SPSS Statistics Version 24. Descriptive analyses were performed to assess patient and tumor characteristics. The diagnostic performance of SR was evaluated by calculating sensitivity and specificity along with 95% confidence intervals. In addition, the positive and negative predictive values are based on the observed sample prevalence. We also provide the number needed to treat (NNT) using histological assessment findings as reference. The Chi-square test was used to assess differences in the the distribution of categorical variables. P values are not adjusted for multiplicity and must be interpreted descriptively.
Results
Patient and tumor characteristics
Mean age was 60.2 years (SD 10.9). Of all 470 lesions, 417 (88.7%) cancers were invasive, 53 (11.3%) in situ carcinoma without invasive component (Table 1). Mean tumor size including DCIS expansion was 18.5 mm (SD 11, range 1–80 mm).
Preoperative wire localization
423 (90%) lesions were preoperatively wire-marked: 358 guided by ultrasound, 61 by mammography, and 4 using both methods. 47 (10%) cases did not receive preoperative wire localization due to palpability.
Histopathological examination
Before the performance of targeted re-resections, a total of 310 (11.0%) margins showed infiltration in the histopathological examination.
This translates to 167 patients (35.5%) on case level. Considering patient and tumor characteristics, the main risk factor for initial margin infiltration was a larger tumor size of 310 infiltrated margins, 69 (22.3%) were located in cranial, whereas the other directions ranged from 44 (14.2%, ventral) to 51 (16.5%, lateral).
SR
SR was performed in all 470 cases. Therefore, 2820 margins were considered (Fig. 3). 445 (15.8%) margins were radiologically positive. Thereof, 114 (4.0%) were assessed correctly (true positive) (Table 2). Medial and lateral orientations showed the highest rate of true positive identification with 46.0% and 45.1%, followed by cranial and caudal orientations with 37.7% and 36.7%. Ventral and dorsal orientations displayed the lowest true positive-rates (31.8% and 21.3%). These correctly identified that margins had the potential to go from initially infiltrated to finally negative if re-resections were carried out adequately.
The remaining 331 (11.7%) margins were falsely assessed as infiltrated (false positive), which translates to 132 (28.1%) patients on case level (Table 2). In these cases, healthy tissue was resected unnecessarily if the surgeon followed the recommendation for re-resection. For these patients SR was disadvantageous.
Of 2375 (84.2%) radiologically negative margins, 2179 (77.3%) were assessed correctly (true negative). 196 (7.0%) were histologically infiltrated (false negative) (Table 2). If not autonomously re-resected by the surgeon, these margins were finally positive which set the indication for further therapy. These two groups of patients had neither a benefit nor a disadvantage by the use of SR.
This results in a sensitivity of 36.8%, a specificity of 86.8%, a PPV of 25.6%, and a NPV of 91.8% (Table 3).
Targeted re-excisions
In total, 431 targeted re-excisions in 291 cases were performed within the primary surgery. In 89.7% of those cases only one or two targeted re-excisions were additionally excised (frequencies as listed in Table 4). On average, 1.5 re-resections per patient who are receiving re-excision were performed. Pathology documented the re-resection weight in 187 of 291 cases. The mean weight additionally excised was 12.3 g (SD 12.1, range 1 to 101 g).
Of all recommended re-resections, re-excision of the ventral and dorsal orientation were refused most frequently, because in the case of maximal surgery up to the fascia respectively the skin, surgery was considered concluded even if margins were histopathologically positive. These cases might wrongfully be registered as “false negative re-resections”.
Final positive margin rate
After consideration of the re-excision-specimens, the final histopathological result was conducted. The number of initially infiltrated margins could be reduced from 310 (11.0%) to 214 (7.6%), i.e., by 30.6% with an absolute risk reduction of 3.4%. This results in a NNT of 30 margins needed to be analyzed and treated to convert one initially positive margin into finally negative.
On case level, 162 (34.5%) patients would have required further surgery in the hypothetical case where no margin assessment and no re-resections would have been carried out. Therefore, SR in addition to gross assessment by the surgeon reduced the rate of secondary procedures by 37.0% (from 162 to 102 (21.7%)).
Secondary procedures
102 cases (21.7%), consisting of 100 cases with a final positive margin status and 2 cases with satellite lesions in pathology, required further surgical therapy to obtain negative margins; 84 breast conserving and 15 secondary mastectomies were conducted. Three patients were operated in another center. Seven cases required a third procedure, five of these were secondary mastectomies. In one case, four surgeries were necessary to excise the malignant tissue completely.
Discussion
SR has been explored in several studies [21,22,23,24,25]. However, to our knowledge, this study is first to assess margins separately. We believe it is necessary to go on a margin level to analyze SR accurately if targeted re-excisions are performed. Otherwise, if a specimen with a radiologically positive margin status proves to show infiltration in histopathology, the SR-finding might be labeled “true positive,” although orientations of radiological and histological involvement diverge.
Even though SR has been an established tool of intraoperative margin assessment for more than two decades, study comparability is low. A recent meta-analysis by Versteegden et al. demonstrated the weaknesses of the existing studies. Sensitivity/specificity and PPV/NPV ranged from 22 to 77%/51 to 100% and 53 to 100%/32 to 95%, respectively [26]. This divergence is based on diverse study structures with varying definitions of key parameters, presenting a challenge in terms of comparison. Prospectively, important steps to generate comparable studies would be an agreement on a consistent definition of a safe margin width, currently ranging from “no tumor on ink” [27, 28] to > 5 mm [22], and the determination on statistical parameters to be calculated, e.g., the number needed to treat.
Multiple studies [29,30,31], including a recent review by Gray et al. [32], indicate that SR is not able to reduce the rate of positive margins and hence, the reoperation rate. Hisada et al. stated that their PMR (positive margin rate) was not affected by re-resections as advised by digital SR [22]. Bimston et al. stated that only 1.8% of patients benefited from its use [33]. Reasons for those results might be the renunciation of a second view in most of these studies.
In our study however, we noticed a reduction of infiltrated margins from 11 to 4%, translating to a reduction of secondary procedures from 34.5 to 21.7% on case level. This is concordant with Ciccarelli et al. and Chagpar et al., who reported a reduction of second surgeries, with their PMR ranging from 31 to 21% [24] and 37.8 to 28.9%, respectively [34]. McCormick et al. stated a reduction from 12 to 5% [21], indicating a generous primary resection as their PMR was remarkably low. It shall be noted that in our study not only SR but also the surgeon’s clinical impression set the indication for re-excision, limiting this study as it caused difficulty in assessing the respective impact on the reduction of second surgeries due to the retrospective nature of this study.
Moreover, we report a high specificity and NPV of 86.8%/91.8%. These results are influenced by a high majority of initially clear margins (89%). Naz et al. explored the ability of SR to predict complete local excision and reported a sensitivity/specificity of 80.7%/81% [35]. Hence, SR is a sensitive tool to provide intraoperative assurance of complete excision, which pathology can only achieve postoperatively.
Specimen orientation
The low sensitivity and PPV of 36.8% and 25.6% are likely to be influenced by errors of orientation and the difficulty of adequate specimen-handling. Schmachtenberg et al. stated incorrect orientations in as many as 16.9% of all cases with correctly diagnosed margin involvement [23]. Molina et al. reported an overall disorientation rate of 31.1%, which especially affected lower volume specimens [36].
A false orientation of the specimen can have serious implications, e.g., re-excision of a false orientation and a remaining positive margin status, putting the patient at risk for local recurrence. Finally, the orientation by pathology is used as gold standard, although it may deviate from the original anatomical position. Regarding the assessment divided by orientation, it is noticeable that there seems to be a division in pairs: medial–lateral and cranial-caudal. This is likely to be attributed by the fact that if an error of orientation occurs, the corresponding direction will also be affected. Prevention and improvements should be made through an immediate and careful marking, as well as great diligence in handling the specimen in the following processes.
False positives
In our study, we report a high rate of 11.7% false positively assessed margins. If re-resected, these patients suffered a disadvantage as healthy tissue was unnecessarily excised. Ota et al. found similar results with a radiological PMR of 47% versus a true positive margin rate of 5.8% [27]. However, this study is limited by a low case count of 17 patients. The pancake phenomenon as stated by Graham et al. provides possible explanation. They found an average loss of the specimen’s height and mean volume after excision of 46% and 30%, resulting in flattened tissue in which malignant tissue might appear falsely close to the resection margin, generating false positive findings. Furthermore, SR uses compression which enhances the effect [37]. Clingan et al. stated that undue specimen compression is common in routine SR and results in potential margin distortion, although it is unnecessary for successful image generation [38]. In the future, efforts should be made to avoid specimen alteration e.g., by fixation methods and renunciation of compression that causes alteration.
We found that the advantage of identifying infiltrated margins outweighs the disadvantage of false positive re-resections in this setting, as targeted re-excisions are usually of very limited volume, particularly in comparison to the cavity shave re-excisions [39, 40]. The median weight additionally excised as documented in 187 cases was 12.3 g, which presumably had only a minor effect on the aesthetic result.
Radiological margin width
To obtain an ideal aesthetic outcome, we removed a minimal amount of tissue. Therefore, margins tend to be narrow and are only considered radiologically positive if the tumor seems to infiltrate the edge. The viewer assesses the specimen without carrying out measurements; this requires high-level expertise and good communication between the mammographer and the surgeon. In the future, measuring of margin distances could be performed to generate more comprehensible results and to achieve comparability.
Studies indicate that a certain radiological margin width is a predictive factor for a positive margin status. Recommendations, as to how wide a radiological margin should be in order to obtain a clear histopathological margin range from 4 to 10 mm in DCIS and 0 to 5 mm in all other cancers [23, 25, 41]. Mazouni et al. stated furthermore that a higher threshold value correlated with higher SR-sensitivity (radiological cut off 1 mm—sensitivity: 33%; 5 mm—50%; 10 mm—75%) [25].
Cavity shave margins
Cavity shave margins are a similar approach in the sense that a higher volume of resected tissue correlates with a lower risk of final infiltration and therefore local recurrence. Huston et al. reported a secondary procedure rate of 17.7% after cavity shaving in comparison to 32.5% after selective re-resection of 1–3 margins [40]. Chagpar et al. stated in their prospective randomized trial that they could reduce the initial PMR in the shave group from 36 to 19%, while the no-shave group, in which patients received standard-protocol BCS including intraoperative margin assessment, had a final PMR of 34% [39].
The renunciation of selectivity such as systematic or cavity shave resections are accompanied by the resection of more tissue-volume [39, 40] and consequently a less ideal aesthetic result, although in their study Chagpar et al. claimed that there was no decrement of patient-perceived cosmeses [39]. This could be due to the usual unfamiliarity of patients with the aesthetic results of BCS and the missing basis for comparison. Brands-Appeldoorn et al. showed that the agreement on aesthetic outcomes after BCS between patients and professionals was only moderate [42], Al-Ghazal et al. even noted in a review that many studies have shown that patients tend to evaluate the aesthetic outcome more positively than health care providers [43]. As cosmesis and quality of life after BCT are closely linked, efforts should be made to assess the aesthetic outcome thoroughly.
It could be argued that the increased excised volume within the primary procedure and the subsequent effect on cosmesis is less severe than a potential secondary intervention, if margin control is not as effective. Gibson et al. explored the local recurrence rate after cavity shaving versus targeted re-resectioning after the histopathological assessment and concluded that ink-directed re-excision of specimens with an initial positive margin status minimized the amount of tissue removed without increasing the incidence of local recurrence and is therefore the preferable method [44]. This shows that if exerted correctly, selective re-resections are a safe method regarding long-term local control.
The complete excision of the tumor must be the goal of BSC. It is clear; the more tissue removed, the lower the risk of remaining malignant tissue—but at what cost for the patient? Current guidelines demonstrate the tendency towards more narrow margins. SR as an intraoperative tool can function as a safety net, enabling to operate as sparingly as possible. We believe the objective of future efforts should be to improve intraoperative margin control as opposed to go back to more radical and invasive methods at the expense of patients.
Limitations
The limitations include the retrospective nature of this study. Furthermore, the impact of gross inspection by the surgeon as implemented can, if not documented precisely, be elusive and difficult to differentiate from SR on case level, which may lead to distortions in the assessment of SR. In our study, we included all histological types. To assess specific diagnostic accuracy, a distinction between tumor histologies should be performed.
Conclusions
SR is a helpful tool to support the approach of tissue-sparing surgery. It provides intraoperative assurance and is able to prevent secondary procedures by identifying infiltrated margins and recommends selective re-excisions. It shall be noted that SR is prone to sources of error such as specimen orientation and handling, resulting in false positive findings and consequently aesthetic and economic disadvantages. In order to use SR effectively, efforts should be made to minimize those sources of error, so the number of patients benefitting from its use will increase. In the future, prospective studies and long-term follow-up data are necessary to further examine the benefit of intraoperative re-excision guided by SR, specifically for each tumor histology.
References
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347(16):1233–1241. https://doi.org/10.1056/NEJMoa022152
Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E (2002) Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 347(16):1227–1232. https://doi.org/10.1056/NEJMoa020989
Hofvind S, Holen A, Aas T, Roman M, Sebuodegard S, Akslen LA (2015) Women treated with breast conserving surgery do better than those with mastectomy independent of detection mode, prognostic and predictive tumor characteristics. Eur J Surg Oncol 41(10):1417–1422. https://doi.org/10.1016/j.ejso.2015.07.002
Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA (2013) Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer 119(7):1402–1411. https://doi.org/10.1002/cncr.27795
Heil J, Holl S, Golatta M, Rauch G, Rom J, Marme F, Gebauer G, Sohn C (2010) Aesthetic and functional results after breast conserving surgery as correlates of quality of life measured by a German version of the Breast Cancer Treatment Outcome Scale (BCTOS). Breast 19(6):470–474. https://doi.org/10.1016/j.breast.2010.05.004
Waljee JF, Hu ES, Ubel PA, Smith DM, Newman LA, Alderman AK (2008) Effect of esthetic outcome after breast-conserving surgery on psychosocial functioning and quality of life. J Clin Oncol 26(20):3331–3337. https://doi.org/10.1200/JCO.2007.13.1375
Kaviani A, Sodagari N, Sheikhbahaei S, Eslami V, Hafezi-Nejad N, Safavi A, Noparast M, Fitoussi A (2013) From radical mastectomy to breast-conserving therapy and oncoplastic breast surgery: a narrative review comparing oncological result, cosmetic outcome, quality of life, and health economy. ISRN Oncol 2013:742462. https://doi.org/10.1155/2013/742462
Al-Ghazal SK, Fallowfield L, Blamey RW (2000) Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur J Cancer 36(15):1938–1943
Hennigs A, Hartmann B, Rauch G, Golatta M, Tabatabai P, Domschke C, Schott S, Schutz F, Sohn C, Heil J (2015) Long-term objective esthetic outcome after breast-conserving therapy. Breast Cancer Res Treat 153(2):345–351. https://doi.org/10.1007/s10549-015-3540-y
Foersterling E, Golatta M, Hennigs A, Schulz S, Rauch G, Schott S, Domschke C, Schuetz F, Sohn C, Heil J (2014) Predictors of early poor aesthetic outcome after breast-conserving surgery in patients with breast cancer: initial results of a prospective cohort study at a single institution. J Surg Oncol 110(7):801–806. https://doi.org/10.1002/jso.23733
Volders JH, Negenborn VL, Haloua MH, Krekel NMA, Jozwiak K, Meijer S, van den Tol PM (2018) Breast-specific factors determine cosmetic outcome and patient satisfaction after breast-conserving therapy: results from the randomized COBALT study. J Surg Oncol 117(5):1001–1008. https://doi.org/10.1002/jso.25012
Hennigs A, Fuchs V, Sinn HP, Riedel F, Rauch G, Smetanay K, Golatta M, Domschke C, Schuetz F, Schneeweiss A, Sohn C, Heil J (2016) Do patients after reexcision due to involved or close margins have the same risk of local recurrence as those after one-step breast-conserving surgery? Ann Surg Oncol 23(6):1831–1837. https://doi.org/10.1245/s10434-015-5067-1
van Maaren MC, de Munck L, de Bock GH, Jobsen JJ, van Dalen T, Linn SC, Poortmans P, Strobbe LJ, Siesling S (2016) 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol 17(8):1158–1170. https://doi.org/10.1016/S1470-2045(16)30067-5
Dahlback C, Manjer J, Rehn M, Ringberg A (2016) Determinants for patient satisfaction regarding aesthetic outcome and skin sensitivity after breast-conserving surgery. World J Surg Oncol 14(1):303. https://doi.org/10.1186/s12957-016-1053-8
Rubio IT, Esgueva-Colmenarejo A, Espinosa-Bravo M, Salazar JP, Miranda I, Peg V (2015) Intraoperative ultrasound-guided lumpectomy versus mammographic wire localization for breast cancer patients after neoadjuvant treatment. Ann Surg Oncol. https://doi.org/10.1245/s10434-015-4935-z
Muttalib M, Tai CC, Briant-Evans T, Maheswaran I, Livni N, Shousha S, Sinnett HD (2005) Intra-operative assessment of excision margins using breast imprint and scrape cytology. Breast 14(1):42–50. https://doi.org/10.1016/j.breast.2004.10.002
Osako T, Nishimura R, Nishiyama Y, Okumura Y, Tashima R, Nakano M, Fujisue M, Toyozumi Y, Arima N (2015) Efficacy of intraoperative entire-circumferential frozen section analysis of lumpectomy margins during breast-conserving surgery for breast cancer. Int J Clin Oncol. https://doi.org/10.1007/s10147-015-0827-2
Kupstas A, Ibrar W, Hayward RD, Ockner D, Wesen C, Falk J (2017) A novel modality for intraoperative margin assessment and its impact on re-excision rates in breast conserving surgery. Am J Surg. https://doi.org/10.1016/j.amjsurg.2017.11.023
American Joint Committee on Cancer (2010) AJCC cancer staging manual, 7th edn. Springer, New York
Mammakarzinom SL (2012) Interdisziplinäre S3-Leitlinie für die Diagnostik, Therapie und Nachsorge des Mammakarzinoms. Leitlinienprogramm Onkologie (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V., Deutsche Krebsgesellschaft, Deutsche Krebshilfe). http://www.awmf.org/uploads/tx_szleitlinien/032045OL_k_S3__Brustkrebs_Mammakarzinom_Diagnostik_Therapie_Nachsorge_2012-07.pdf. Accessed 6 May 2019
McCormick JT, Keleher AJ, Tikhomirov VB, Budway RJ, Caushaj PF (2004) Analysis of the use of specimen mammography in breast conservation therapy. Am J Surg 188(4):433–436. https://doi.org/10.1016/j.amjsurg.2004.06.030
Hisada T, Sawaki M, Ishiguro J, Adachi Y, Kotani H, Yoshimura A, Hattori M, Yatabe Y, Iwata H (2016) Impact of intraoperative specimen mammography on margins in breast-conserving surgery. Mol Clin Oncol 5(3):269–272. https://doi.org/10.3892/mco.2016.948
Schmachtenberg C, Engelken F, Fischer T, Bick U, Poellinger A, Fallenberg EM (2012) Intraoperative specimen radiography in patients with nonpalpable malignant breast lesions. Rofo 184(7):635–642. https://doi.org/10.1055/s-0032-1312730
Ciccarelli G, Di Virgilio MR, Menna S, Garretti L, Ala A, Giani R, Bussone R, Canavese G, Berardengo E (2007) Radiography of the surgical specimen in early stage breast lesions: diagnostic reliability in the analysis of the resection margins. Radiol Med 112(3):366–376. https://doi.org/10.1007/s11547-007-0147-3
Mazouni C, Rouzier R, Balleyguier C, Sideris L, Rochard F, Delaloge S, Marsiglia H, Mathieu MC, Spielman M, Garbay JR (2006) Specimen radiography as predictor of resection margin status in non-palpable breast lesions. Clin Radiol 61(9):789–796. https://doi.org/10.1016/j.crad.2006.04.017
Versteegden DPA, Keizer LGG, Schlooz-Vries MS, Duijm LEM, Wauters CAP, Strobbe LJA (2017) Performance characteristics of specimen radiography for margin assessment for ductal carcinoma in situ: a systematic review. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-017-4475-2
Ota K, Rivera C, Martin M (2017) Specimen mammography distorts margin status in patients undergoing breast conserving surgery for early-stage breast cancer. Breast J 23(6):760–761. https://doi.org/10.1111/tbj.12920
Ihrai T, Quaranta D, Fouche Y, Machiavello JC, Raoust I, Chapellier C, Maestro C, Marcy M, Ferrero JM, Flipo B (2014) Intraoperative radiological margin assessment in breast-conserving surgery. Eur J Surg Oncol 40(4):449–453. https://doi.org/10.1016/j.ejso.2014.01.002
Rua C, Lebas P, Michenet P, Ouldamer L (2012) Evaluation of lumpectomy surgical specimen radiographs in subclinical, in situ and invasive breast cancer, and factors predicting positive margins. Diagn Interv Imaging 93(11):871–877. https://doi.org/10.1016/j.diii.2012.07.010
Laws A, Brar MS, Bouchard-Fortier A, Leong B, Quan ML (2018) Does intra-operative margin assessment improve margin status and re-excision rates? A population-based analysis of outcomes in breast-conserving surgery for ductal carcinoma in situ. J Surg Oncol 118(7):1205–1211. https://doi.org/10.1002/jso.25248
Laws A, Brar MS, Bouchard-Fortier A, Leong B, Quan ML (2016) Intraoperative margin assessment in wire-localized breast-conserving surgery for invasive cancer: a population-level comparison of techniques. Ann Surg Oncol 23(10):3290–3296. https://doi.org/10.1245/s10434-016-5401-2
Gray RJ, Pockaj BA, Garvey E, Blair S (2017) Intraoperative margin management in breast-conserving surgery: a systematic review of the literature. Ann Surg Oncol. https://doi.org/10.1245/s10434-016-5756-4
Bimston DN, Bebb GG, Wagman LD (2000) Is specimen mammography beneficial? Arch Surg 135(9):1083–1086 (discussion 1086-1089)
Chagpar AB, Butler M, Killelea BK, Horowitz NR, Stavris K, Lannin DR (2015) Does three-dimensional intraoperative specimen imaging reduce the need for re-excision in breast cancer patients? A prospective cohort study. Am J Surg 210(5):886–890. https://doi.org/10.1016/j.amjsurg.2015.05.018
Naz S, Masroor I, Afzal S, Mirza W, Butt S, Sajjad Z, Ahmad A (2018) Accuracy of specimen radiography in assessing complete local excision with breast-conservation surgery. Asian Pac J Cancer Prev 19(3):763–767. https://doi.org/10.22034/APJCP.2018.19.3.763
Molina MA, Snell S, Franceschi D, Jorda M, Gomez C, Moffat FL, Powell J, Avisar E (2009) Breast specimen orientation. Ann Surg Oncol 16(2):285–288. https://doi.org/10.1245/s10434-008-0245-z
Graham RA, Homer MJ, Katz J, Rothschild J, Safaii H, Supran S (2002) The pancake phenomenon contributes to the inaccuracy of margin assessment in patients with breast cancer. Am J Surg 184(2):89–93
Clingan R, Griffin M, Phillips J, Coberly W, Jennings W (2003) Potential margin distortion in breast tissue by specimen mammography. Arch Surg 138(12):1371–1374. https://doi.org/10.1001/archsurg.138.12.1371
Chagpar AB, Killelea BK, Tsangaris TN, Butler M, Stavris K, Li F, Yao X, Bossuyt V, Harigopal M, Lannin DR, Pusztai L, Horowitz NR (2015) A randomized, controlled trial of cavity shave margins in breast cancer. N Engl J Med 373(6):503–510. https://doi.org/10.1056/NEJMoa1504473
Huston TL, Pigalarga R, Osborne MP, Tousimis E (2006) The influence of additional surgical margins on the total specimen volume excised and the reoperative rate after breast-conserving surgery. Am J Surg 192(4):509–512. https://doi.org/10.1016/j.amjsurg.2006.06.021
Lange M, Reimer T, Hartmann S, Glass A, Stachs A (2016) The role of specimen radiography in breast-conserving therapy of ductal carcinoma in situ. Breast 26:73–79. https://doi.org/10.1016/j.breast.2015.12.014
Brands-Appeldoorn A, Maaskant-Braat AJG, Zwaans WAR, Dieleman JP, Schenk KE, Broekhuysen CL, Weerdenburg H, Daniels R, Tjan-Heijnen VCG, Roumen RMH (2018) Patient-reported outcome measurement compared with professional judgment of cosmetic results after breast-conserving therapy. Curr Oncol 25(6):e553–e561. https://doi.org/10.3747/co.25.4036
Al-Ghazal SK, Blamey RW (1999) Cosmetic assessment of breast-conserving surgery for primary breast cancer. Breast 8(4):162–168. https://doi.org/10.1054/brst.1999.0017
Gibson GR, Lesnikoski BA, Yoo J, Mott LA, Cady B, Barth RJ Jr (2001) A comparison of ink-directed and traditional whole-cavity re-excision for breast lumpectomy specimens with positive margins. Ann Surg Oncol 8(9):693–704
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the University of Heidelberg ethics committee (S-468/2016) and in accordance with the Declaration of Helsinki.
Informed consent
Patient consent was waived as the study did not interfere with standard-of-care nor did it disclose or analyze protected health information.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Funk, A., Heil, J., Harcos, A. et al. Efficacy of intraoperative specimen radiography as margin assessment tool in breast conserving surgery. Breast Cancer Res Treat 179, 425–433 (2020). https://doi.org/10.1007/s10549-019-05476-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05476-6